 | |
| Names | |
|---|---|
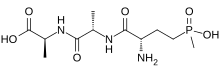
| Systematic IUPAC name
(2S)-2-[(2S)-2-{(2S)-2-Amino-4-[hydroxy(methylphosphonoyl)]butanamido}propanamido]propanoic acid | |
| Other names
L-Alanyl-L-alanyl-phosphinothricin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.113.731 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H22N3O6P | |
| Molar mass | 323.286 g·mol−1 |
| Density | 1.33 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bialaphos is a natural herbicide produced by the bacteria Streptomyces hygroscopicus[1] and Streptomyces viridochromogenes. It is also known by the ISO common name bilanafos.[2] Bialaphos is a protoxin and nontoxic as is. When it is metabolized by a plant, the glutamic acid analog glufosinate is released which inhibits glutamine synthetase. This results in the accumulation of ammonium and disruption of primary metabolism.[3]
Bialaphos is made up of two alanine residues and glufosinate, and is commonly used as a selection marker in plants. Resistance plasmids include pGreenII 0229 and pGreenII 0229 62-SK. pGreenII 0229 is derived from pGreenII 0000, a nos-bar cassette has been inserted into the HpaI site of the left border, providing resistance to bialaphos or phosphinothricin during plant transformation selection. pGreenII 0229 62-SK is derived from pGreenII 0229, the LacZ blue/white cloning selection has been replaced with a 35S-MCS-CaMV cassette that allows the insertion of a gene of interest into a 35S overexpression cassette.[4]
See also
[edit]- Phosalacine, a related tripeptide
References
[edit]- ^ Murakami, Takeshi; Anzai, Hiroyuki; Imai, Satoshi; Satoh, Atsuyuki; Nagaoka, Kozo; Thompson, Charles J. (1986). "The bialaphos biosynthetic genes of Streptomyces hygroscopicus: Molecular cloning and characterization of the gene cluster". MGG Molecular & General Genetics. 205: 42–53. doi:10.1007/BF02428031. S2CID 32983239.
- ^ "Compendium of Pesticide Common Names: Bilanafos". BCPC. Retrieved 2024-05-07.
- ^ Duke, Stephen O.; Dayan, Franck E. (2011). "Modes of Action of Microbially-Produced Phytotoxins". Toxins (Basel). 3 (8): 1038–1064. CiteSeerX 10.1.1.288.3457. doi:10.3390/toxins3081038. PMC 3202864. PMID 22069756.
- ^ "Bialaphos as plant gene selector" (PDF). Archived from the original (PDF) on 21 October 2014. Retrieved 20 June 2012.