| fibrillin 1 | |||||||
|---|---|---|---|---|---|---|---|
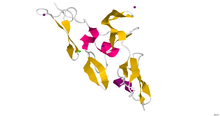 Crystallographic structure of the cbEGF9-hybrid2-cbEGF10 region of human fibrillin 1.[1] | |||||||
| Identifiers | |||||||
| Symbol | FBN1 | ||||||
| Alt. symbols | FBN, MFS1, WMS | ||||||
| NCBI gene | 2200 | ||||||
| HGNC | 3603 | ||||||
| OMIM | 134797 | ||||||
| PDB | 2W86 | ||||||
| RefSeq | NM_000138 | ||||||
| UniProt | P35555 | ||||||
| Other data | |||||||
| Locus | Chr. 15 q21.1 | ||||||
| |||||||
| fibrillin 2 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | FBN2 | ||||||
| Alt. symbols | CCA | ||||||
| NCBI gene | 2201 | ||||||
| HGNC | 3604 | ||||||
| OMIM | 121050 | ||||||
| RefSeq | NM_001999 | ||||||
| UniProt | P35556 | ||||||
| Other data | |||||||
| Locus | Chr. 5 q23-q31 | ||||||
| |||||||
| fibrillin 3 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | FBN3 | ||||||
| NCBI gene | 84467 | ||||||
| HGNC | 18794 | ||||||
| OMIM | 608529 | ||||||
| RefSeq | NM_032447 | ||||||
| UniProt | Q75N90 | ||||||
| Other data | |||||||
| Locus | Chr. 19 p13 | ||||||
| |||||||
Fibrillin is a glycoprotein, which is essential for the formation of elastic fibers found in connective tissue.[2] Fibrillin is secreted into the extracellular matrix by fibroblasts and becomes incorporated into the insoluble microfibrils, which appear to provide a scaffold for deposition of elastin.[3]
Clinical aspects
[edit]Marfan syndrome is a genetic disorder of the connective tissue caused by defected FBN1 gene. Mutations in FBN1 and FBN2 are also sometimes associated with adolescent idiopathic scoliosis.[4]
Types
[edit]Fibrillin-1
[edit]Fibrillin-1 is a major component of the microfibrils that form a sheath surrounding the amorphous elastin. It is believed that the microfibrils are composed of end-to-end polymers of fibrillin. To date, 3 forms of fibrillin have been described. The fibrillin-1 protein was isolated by Engvall in 1986,[5] and mutations in the FBN1 gene cause Marfan syndrome.[6][7]
This protein is found in humans, and its gene is found on chromosome 15. At present more than 1500 different mutations have been described.[1][7]
Structure
[edit]There is no complete, high-resolution structure of fibrillin-1. Instead, short fragments have been produced recombinantly and their structures solved by X-ray crystallography or using NMR spectroscopy. A recent example is the structure of the fibrillin-1 hybrid2 domain, in context of its flanking calcium binding epidermal growth factor domains, which was determined using X-ray crystallography to a resolution of 1.8 Å.[1] The microfibrils that are made up of fibrillin protein are responsible for different cell-matrix interactions in the human body.
Fibrillin-2
[edit]Fibrillin-2 was isolated in 1994 by Zhang[8] and is thought to play a role in early elastogenesis. Mutations in the fibrillin-2 gene have been linked to Beals syndrome.
Fibrillin-3
[edit]More recently, fibrillin-3 was described and is believed to be located mainly in the brain.[9] Along with the brain, fibrillin-3 has been localized in the gonads and ovaries of field mice.
Fibrillin-4
[edit]Fibrillin-4 was first discovered in zebrafish, and has a sequence similar to fibrillin-2.[10]
References
[edit]- ^ a b c PDB: 2W86; Jensen SA, Iqbal S, Lowe ED, Redfield C, Handford PA (May 2009). "Structure and interdomain interactions of a hybrid domain: a disulphide-rich module of the fibrillin/LTBP superfamily of matrix proteins". Structure. 17 (5): 759–68. doi:10.1016/j.str.2009.03.014. PMC 2724076. PMID 19446531.
- ^ Kielty CM, Baldock C, Lee D, Rock MJ, Ashworth JL, Shuttleworth CA (February 2002). "Fibrillin: from microfibril assembly to biomechanical function". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 357 (1418): 207–17. doi:10.1098/rstb.2001.1029. PMC 1692929. PMID 11911778.
- ^ Singh (2006). Textbook of human histology. Jaypee Brothers Publishers. pp. 64–. ISBN 978-81-8061-809-3. Retrieved 9 December 2010.[permanent dead link]
- ^ Buchan JG, Alvarado DM, Haller GE, Cruchaga C, Harms MB, Zhang T, Willing MC, Grange DK, Braverman AC, Miller NH, Morcuende JA, Tang NL, Lam TP, Ng BK, Cheng JC, Dobbs MB, Gurnett CA (October 2014). "Rare variants in FBN1 and FBN2 are associated with severe adolescent idiopathic scoliosis". Human Molecular Genetics. 23 (19): 5271–82. doi:10.1093/hmg/ddu224. PMC 4159151. PMID 24833718.
- ^ Sakai LY, Keene DR, Engvall E (December 1986). "Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils". The Journal of Cell Biology. 103 (6 Pt 1): 2499–509. doi:10.1083/jcb.103.6.2499. PMC 2114568. PMID 3536967.
- ^ Dietz HC (August 2010). "New therapeutic approaches to mendelian disorders". The New England Journal of Medicine. 363 (9): 852–63. doi:10.1056/NEJMra0907180. PMID 20818846.
- ^ a b von Kodolitsch Y, De Backer J, Schüler H, Bannas P, Behzadi C, Bernhardt AM, Hillebrand M, Fuisting B, Sheikhzadeh S, Rybczynski M, Kölbel T, Püschel K, Blankenberg S, Robinson PN (2015). "Perspectives on the revised Ghent criteria for the diagnosis of Marfan syndrome". The Application of Clinical Genetics. 8: 137–55. doi:10.2147/TACG.S60472. PMC 4476478. PMID 26124674.
- ^ Zhang H, Apfelroth SD, Hu W, Davis EC, Sanguineti C, Bonadio J, Mecham RP, Ramirez F (March 1994). "Structure and expression of fibrillin-2, a novel microfibrillar component preferentially located in elastic matrices". The Journal of Cell Biology. 124 (5): 855–63. doi:10.1083/jcb.124.5.855. PMC 2119952. PMID 8120105.
- ^ Corson GM, Charbonneau NL, Keene DR, Sakai LY (March 2004). "Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues". Genomics. 83 (3): 461–72. doi:10.1016/j.ygeno.2003.08.023. PMID 14962672.
- ^ Gansner JM, Madsen EC, Mecham RP, Gitlin JD (October 2008). "Essential role for fibrillin-2 in zebrafish notochord and vascular morphogenesis". Developmental Dynamics. 237 (10): 2844–61. doi:10.1002/dvdy.21705. PMC 3081706. PMID 18816837.
External links
[edit]- Fibrillin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)