 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic (CYP2C19-mediated) |
| Elimination half-life | 4.8 to 7.7 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.120.697 |
| Chemical and physical data | |
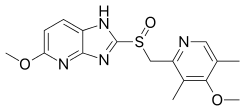
| Formula | C16H18N4O3S |
| Molar mass | 346.41 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Tenatoprazole is a proton pump inhibitor drug candidate that was undergoing clinical testing as a potential treatment for reflux oesophagitis and peptic ulcer as far back as 2003.[1] The compound was invented by Mitsubishi Tanabe Pharma and was licensed to Negma Laboratories (part of Wockhardt as of 2007[2]).[3]: 22
Mitsubishi reported that tenatoprazole was still in Phase I clinical trials in 2007[4]: 27 and again in 2012.[3]: 17
Tenatoprazole has an imidazopyridine ring in place of the benzimidazole moiety found in other proton pump inhibitors, and has a half-life about seven times longer than other PPIs.[5]
See also
[edit]References
[edit]- ^ "Gastrointestinal Disease Update". Digestive Disease Week. DataMonitor. March 2003.
- ^ "Investors unwilling to forgive Wockhardt, promoter for failings". Economic Times. 3 March 2011. Archived from the original on November 16, 2014.
- ^ a b "State of New Product Development" (PDF). Mitsubishi Tanabe Pharma. 8 May 2012.
- ^ "FY2007 Interim Financial Results". Mitsubishi Tanabe Pharma.
- ^ Li H, Meng L, Liu F, Wei JF, Wang YQ (January 2013). "H+/K+-ATPase inhibitors: a patent review". Expert Opinion on Therapeutic Patents. 23 (1): 99–111. doi:10.1517/13543776.2013.741121. PMID 23205582. S2CID 44647770.