| Toolbox |
|---|
This peer review discussion has been closed.
Main objectives:
1. Footnote format: find issues and fix them. Whole article. Thanks for hands on help.
2. Review of the wikilinks: stem to stern first linking...prefer very low density of blue bumps, dabs, all that).
3. Standard: prose, content, expert review, non-expert, images, boxes/cats/templates. Since it is long, section reviews are fine. For prose glitches or formatting, just fix them please.
-TCO
- Will have to be scarce during the week, please carry on.
- All of you, people, who have taken your time or plan to do so to check this article, thank you very much.--R8R Gtrs (talk) 17:02, 8 October 2013 (UTC)
- May need to be away some time with work and with a looming death in family. Please excuse any ignoring, if I'm scarce. Appreciate all the work so far and I see Wehwalt and Axl still working on topic. When complete, will close the review and thank all participants.208.44.87.91 (talk) 00:39, 24 October 2013 (UTC)
Nikki
[edit]- File:Happy_Pan_Poster.jpg is pretty clearly not the uploader's own work - any idea of source or licensing?
- Had similar concern. See [1]. Should I get CLindberg to do a registration search? Scrap it?
- If you can find more info it'd be great, but if not you'll probably have to scrap it. Nikkimaria (talk) 17:55, 6 October 2013 (UTC)
- cut from article and nommed for deletion at commons.
- If you can find more info it'd be great, but if not you'll probably have to scrap it. Nikkimaria (talk) 17:55, 6 October 2013 (UTC)
- Had similar concern. See [1]. Should I get CLindberg to do a registration search? Scrap it?
- What's a "crash program"?
- [2]
- An "[intense course of training or research] was making significant quantities of Teflon"? Not sure that makes sense. Nikkimaria (talk) 18:58, 6 October 2013 (UTC)
- I think it's normal usage, but I reworded.
- An "[intense course of training or research] was making significant quantities of Teflon"? Not sure that makes sense. Nikkimaria (talk) 18:58, 6 October 2013 (UTC)
- [2]
- What are the page numbers supposed to be for Barrett 1967? Nikkimaria (talk) 17:19, 6 October 2013 (UTC)
- 740-743
- What makes askthenerd and gdrc.org reliable?
- Kill askthenerd. Here are substitutes, please. I think the first is best (since the point is trivial, a textbook is the clearest explanation): [3], [4], [5].
- I can't find gdrc.org in article. Which link is it?
- Currently FN195. Nikkimaria (talk) 18:58, 6 October 2013 (UTC)
- Please kill gdrc.org and add pages 208-216 to the IPCC reference (it covers the content, just very long so I want page number specified).
- Currently FN195. Nikkimaria (talk) 18:58, 6 October 2013 (UTC)
- There are a couple of {{cite doi}} awaiting automatic expansion, but with the deny-citationbot tag they're not going to be automatically expanded. Nikkimaria (talk) 17:58, 6 October 2013 (UTC)
- Help? :( (I don't even like doi cruft.)
StringTheory11
[edit]- Probably better to move the compounds section to between the characteristics and occurrences section.
- Please no. That is is a structural review of the compounds. The general reader can understand the development fine and the "pill" of all that techie content at the front does more damage than help. I am fighting for making technical articles accessible. Chemistry of the Elements has the same structure to put the hard core chemistry at the back.
- My thoughts were that it would make sense for compounds to come before applications, so that some of the compounds discussed there can be introduced. If you want to do it some other way, though, that's fine by me. StringTheory11 (t • c) 02:11, 7 October 2013 (UTC)
- Only way to know if your way works is to try it. Done.
- My thoughts were that it would make sense for compounds to come before applications, so that some of the compounds discussed there can be introduced. If you want to do it some other way, though, that's fine by me. StringTheory11 (t • c) 02:11, 7 October 2013 (UTC)
- Please no. That is is a structural review of the compounds. The general reader can understand the development fine and the "pill" of all that techie content at the front does more damage than help. I am fighting for making technical articles accessible. Chemistry of the Elements has the same structure to put the hard core chemistry at the back.
- I think there may be too many images, especially galleries. They, at least to me, disrupt the flow of the article, and we should cut back on some of them.
- killed several images and galleries and shrank the pictures.
- Any information on the production of non-natural fluorine compounds, not just fluorine gas?
- I added a para on ECF/CoF3 with fluorocarbon industry and a sentence on fluoropolymer production.
More to come later. StringTheory11 (t • c) 19:13, 6 October 2013 (UTC)
LudicrousTripe
[edit]I live for tedious repetitive Wikipedia tasks! I will {{Harvnb}} the shit out of them!!! Cheers! LudicrousTripe (talk) 19:35, 6 October 2013 (UTC)
- thank you. I like the LDR aspect from the use of these shorter templates (less cruft in edit mode)71.127.137.171 (talk) 22:20, 6 October 2013 (UTC)
- aside from that, what are the advantages of this system? (I'm not criticizing, I wanna know)--R8R Gtrs (talk) 19:50, 8 October 2013 (UTC)
- Not really sure, though perhaps others know. Personally, I just like {{Harv}}ing the references because it looks wheel pritty and it's how it's done it t3h real-life books!!?!?!?!?!? Adieu! LudicrousTripe (talk) 08:57, 9 October 2013 (UTC)
Wehwalt
[edit]- "A growing fraction of modern pharmaceuticals contain fluorine" Where is it supported in the body that the percentage is increasing? (I guess that is what "growing fraction" means) I see the use, I don't see the trend.
- Trend was there before, but got trimmed for length. Let's rewrite lead to say "a large minority".
- Significant percentage, maybe?--Wehwalt (talk) 02:02, 7 October 2013 (UTC)
- Perfect.71.127.137.171 (talk) 02:16, 7 October 2013 (UTC)
- Significant percentage, maybe?--Wehwalt (talk) 02:02, 7 October 2013 (UTC)
- Trend was there before, but got trimmed for length. Let's rewrite lead to say "a large minority".
- The definition of fluoride seems rather lost and isolated and I question its presence where it is within the lede. It strikes me it is better handled when the term is first used, perhaps as a parenthetical or clause.
- OK, cut it. [I was pimping the subarticle (linked) and also trying to get an "easy" idea up into the top para...but no big attachment, guess it didn't work.]
- The lede strikes me as rather jumbled. Is there a system you are using to decide what is in what order?--
- The lead is very important to the reader and I sweated it, so your comments make me sad. But...that is why I wanted the thing murder boarded. The structure is (1) characteristics of the element itself (2) occurrence which segues into extraction and then history (3) industry and applications, (4) biological aspects. [A few aspects like hazards, environmental, as well as a touch of the structural compound review are added where they seem to fit...mostly within 3.] Please feel free to do a fundamental re-org. Only way to know if there is a better structure is to try it. (P.s. thank you)71.127.137.171 (talk) 00:18, 7 October 2013 (UTC)
- Hydrofluoric acid is a weak acid in terms of chemical strength, so "powerful" is a misnomer. HF is noteworthy for its corrosive nature (it eats through glass) as well as the poison danger from skin contact (I believe the 19th century injuries were actually mostly from working with HF). We could also just not bother describing HF's features in lead.
- Dangerous was used twice in the lede, one seems to be the max. Pick your word. I did notice the way you've packed the lede with words that are strong, don't know if I like that or not.--Wehwalt (talk) 12:59, 7 October 2013 (UTC)
- Cut both usages. Added poisonous (F2) closer to the front of lead.
- I'm going to add a little more to the burn section to clarify the weakbutdangerous versus strongbutnotsodangerous, more. Doesn't help us that much in lead, but just given both you and Axl had this confusion, it is worthy to call it out. I think we had more of that, but I tightened the thing a lot a while ago and it got harder to grasp with less step by step explanations.
- Cut both usages. Added poisonous (F2) closer to the front of lead.
- Dangerous was used twice in the lede, one seems to be the max. Pick your word. I did notice the way you've packed the lede with words that are strong, don't know if I like that or not.--Wehwalt (talk) 12:59, 7 October 2013 (UTC)
- There was a chronological flow in the second para (formation within stars, deposition in the Earth, extraction and naming of the mineral, isolation of the pure element, and use of the pure element), that also matches the order in the article.
Wehwalt (talk) 00:00, 7 October 2013 (UTC)
- I am starved for time and will look in in short amounts. Why do you want to say STP conditions for the appearance? I understand the significance of STP, but isn't that going to be a stopper for a lot of people? If it doesn't make it totally plebeian, what about room temperature? After all, it doesn't matter what room, if the person in it is still alive, the fluorine is a yellowish gas.--Wehwalt (talk) 02:07, 7 October 2013 (UTC)
- Violent agreement. Hate a techie term when a common one will work.71.127.137.171 (talk) 02:16, 7 October 2013 (UTC)
- Any thoughts on the changes to the lede?
- I like how you moved the basic description to the second sentence. Not crazy about the arrangement of second para (what is the rationale for order of the sentences?) I think you have to try different things and I have to stay away from it and let you play with it, though.
- I felt there was cohesion in the arrangement I have. Start with the general (universe), move to the specific (Earth's crust) and then to the even more specific (mineral). Then we have a comparison with the element, which allows us to move back to discussing the element. Since fluorite was involved in the discovery (the stoppers and so forth), it's defensible. I feel it flows. If you don't agree, change it.--Wehwalt (talk) 21:24, 11 October 2013 (UTC)
- Looks good now, but I'm not totally following you (that's what I wanted, you had something different with history in front of occurrence)208.44.87.91 (talk) 22:46, 14 October 2013 (UTC)
- I'm not a trained writer, and lack the terminology. I just rearranged your sentences until I found an arrangement that seemed to work.--Wehwalt (talk) 01:08, 17 October 2013 (UTC)
- Looks good now, but I'm not totally following you (that's what I wanted, you had something different with history in front of occurrence)208.44.87.91 (talk) 22:46, 14 October 2013 (UTC)
- I felt there was cohesion in the arrangement I have. Start with the general (universe), move to the specific (Earth's crust) and then to the even more specific (mineral). Then we have a comparison with the element, which allows us to move back to discussing the element. Since fluorite was involved in the discovery (the stoppers and so forth), it's defensible. I feel it flows. If you don't agree, change it.--Wehwalt (talk) 21:24, 11 October 2013 (UTC)
- I saw your edit comment about the 99%. Difficult basically means expensive (not something that is not technically understood), but can also be thought of as difficult in terms of cautions, special materials, etc. Consider that PVC is routinely made from chlorine gas (a massive commodity), but PTFE is made from HF. Also, the sources tend to make a big point about how little fluorine is converted to the element.108.162.44.194 (talk) 06:35, 8 October 2013 (UTC)
- Possibly there's also no great demand for elemental fluorine in that form. Just commenting.--Wehwalt (talk) 21:26, 11 October 2013 (UTC)
- I'll look in again as I have time, which may not be until after the weekend.--Wehwalt (talk) 14:19, 11 October 2013 (UTC)
- I've ce d the first two sections, and will continue in that way, leaving hidden comments. If I feel there's something that would benefit from being brought here, I will.--Wehwalt (talk) 21:45, 11 October 2013 (UTC)
- Alright, here's something. I think there is the need for the occasional brief lay explanation, perhaps saying something along the lines (where you discuss F adding an electron), that (suggestion) it readily combines with atoms of other elements. And where you discuss difluorine and its weak bond, make it clear (in your own language) that the fact that the bonds are so easily disrupted means that difluorine is not found naturally on Earth. Sorry if I make any chemical gaffes, I haven't really studied this stuff since high school (I passed the AP exam in chemistry and so did not take it in college).--Wehwalt (talk) 21:58, 11 October 2013 (UTC)
- I've added the explanations and thanks for your edits.98.117.75.177 (talk) 15:39, 12 October 2013 (UTC)
- Not a problem. I only wish I had more time for this, but I'll catch up with it sooner or later.--Wehwalt (talk) 00:57, 17 October 2013 (UTC)
- I've added the explanations and thanks for your edits.98.117.75.177 (talk) 15:39, 12 October 2013 (UTC)
- I like how you moved the basic description to the second sentence. Not crazy about the arrangement of second para (what is the rationale for order of the sentences?) I think you have to try different things and I have to stay away from it and let you play with it, though.
- Any thoughts on the changes to the lede?
- Violent agreement. Hate a techie term when a common one will work.71.127.137.171 (talk) 02:16, 7 October 2013 (UTC)
- In the infobox, what is going on with "reference [12]"? Can't you think of something more useful to do with that space?--Wehwalt (talk) 01:08, 17 October 2013 (UTC)
- I put a note at the template page. Can't edit it. I also want the length of the infobox reduced. For instance some of the icon graphics are duplicative, not worthy of a data table, and some of the info (crystal structures) not high value for a RT gas. I really don't control it (more the opposite).208.44.87.91 (talk) 12:15, 17 October 2013 (UTC)
- Just below the Nobel citation, an image illustrates a "cell". I do not see that term used in the description of the discovery of F. I suggest you either use it in such a way that the reader knows what it is, before the reader encounters the image, or that you make an appropriate link for "cell".--Wehwalt (talk) 01:11, 17 October 2013 (UTC)
- " he was unable to release the gas although the weight had not changed." This isn't written entirely clearly.--Wehwalt (talk) 01:17, 17 October 2013 (UTC)
Wrote a little more explication. Feel free to brush up, cut things, etc.208.44.87.91 (talk) 02:46, 17 October 2013 (UTC)
- It looks OK. I'm down to the end of noble gas, it's very sold.--Wehwalt (talk) 02:47, 20 October 2013 (UTC)
Thanks, big guy. Watch out, one of the po-lice will accuse you of a Freudian. ;-) 208.44.87.91 (talk) 23:25, 22 October 2013 (UTC)
- Small molecules
- "However, the functional group is available for reactions or may make the molecule behave as a surfactant." It's Greek to me.
- Explained this more. (BTW, what would you think of moving compounds to article end?)
- Industry and applications
- "extracting 4.5 million tons per year" You may need to clarify whether this is Imperial or metric units.
- Specified and added back some content (the year was bugging me) and then changed the flow a little as it bugged me too.
- Inorganic fluorides
- In the making of aluminum, if most of the fluorides are recovered for reuse, I would say so.
- I had, but now explained more. :)
- Refrigerant gases
- I think you need to make it clearer to the reader which of these is what he thinks of as "Freon". For people who turn directly to this section
- I added a little more, including a note and also went and redid the flow to be better. You are under a misconception that "Freon"=CFC, but it doesn't. Not just from a geeky thing of DuPont marketing some HFC substitutes as Freon but from people using the word colloquially (still) to refer to the refrigerants in common use (now HFCs and HCFCs).208.44.87.91 (talk) 02:38, 24 October 2013 (UTC)
- Read what I wrote. I am noting the misconception, not making it.--Wehwalt (talk) 22:11, 24 October 2013 (UTC)
- Fluoride ions
- Toothpaste. This begs for expansion with something like "though few cases require medical attention" or whatever it must say". Come on, human interest.
- I'll add a couple epidemiology papers in there. I really don't want the nutters or the "fight the nutters" types in here. Or to open too many doors I can't close, but OK.208.44.87.91 (talk) 02:45, 25 October 2013 (UTC)
- Alright, that's all I've got. It looks very good. I'll give it another read during the FAC.--Wehwalt (talk) 23:03, 24 October 2013 (UTC)
Thanks man.208.44.87.91 (talk) 02:45, 25 October 2013 (UTC)
Jimfbleak
[edit]I'll have a proper look when I get time, just a couple of things for now Jimfbleak - talk to me? 15:26, 7 October 2013 (UTC)
- Lipitor and Prozac— why are the commercial names preferred to generic?
- I want to use the terms that mean the most to the reader. Similarly, I eschew parentheticals because the exec summary should be direct. The article body has the additional detail. Same applies for Teflon. -TCO
- I've bent on this and gone with the parenthetical (say both) approach, given two people are put off. My concern is the readers, readers, readers. Think Wiki has a Caesar's wife fear of appearing commercial as well as a bit of faux academic stiffness. But the whole thing was no big deal and in any case, O acceded.
- I want to use the terms that mean the most to the reader. Similarly, I eschew parentheticals because the exec summary should be direct. The article body has the additional detail. Same applies for Teflon. -TCO
- Fluorine's outer electrons are relatively separate from each other. Hence, they do not shield each other from the nucleus—I'm not convinced by this. The effective nuclear charge increases because there are more protons in the nucleus, but only the same number of inner, screening, shells. It's true that electrons in the same shell don't shield each other, but that misses the main point
- I've rewritten this based on Wehwalt's comments to include more step by step explication.71.127.137.171 (talk) 18:46, 12 October 2013 (UTC)
(thanks in advance)
- decide whether to do conversions for masses. You have 23 kg (51 lb), but metric tonnes later are unconverted (I don't mind, just need consistency)
- Can we discuss this? (All peeps)? I knew this was coming. The conversion of metric tons to short tons is 1.10 (to long is 0.98!). Given the inherent swaggish accuracy of the data (market estimates), I hate to clutter up the prose with a bunch of parenthetical numbers. Really hate slowing the reader with cruft. Anyhow, I could do some cheesy note to this effect at first usage of ton. Or I could just put the parens in. Or I could strip all conversions. And note the issue comes up with temperatures too. I sorta liked having them at the front for phase changes, but then really hate having to have them in the compounds section. Actually my inclination is to strip them all out. It's a science article, not geography or weather. I'm a red white and blue hunter killer and even I'm fine with metric in a chemistry article. I think I will just strip. You all need to back me up if someone gets unhappy they are gone. ;-)71.127.137.171 (talk) 08:16, 13 October 2013 (UTC)
- I stripped them all. If I missed one, fix pls.
- Can we discuss this? (All peeps)? I knew this was coming. The conversion of metric tons to short tons is 1.10 (to long is 0.98!). Given the inherent swaggish accuracy of the data (market estimates), I hate to clutter up the prose with a bunch of parenthetical numbers. Really hate slowing the reader with cruft. Anyhow, I could do some cheesy note to this effect at first usage of ton. Or I could just put the parens in. Or I could strip all conversions. And note the issue comes up with temperatures too. I sorta liked having them at the front for phase changes, but then really hate having to have them in the compounds section. Actually my inclination is to strip them all out. It's a science article, not geography or weather. I'm a red white and blue hunter killer and even I'm fine with metric in a chemistry article. I think I will just strip. You all need to back me up if someone gets unhappy they are gone. ;-)71.127.137.171 (talk) 08:16, 13 October 2013 (UTC)
- Within Earth's crust, fluorine is the thirteenth most abundant— I'd put "the Earth and not hyphenate
- Done
- outside of temporary existence in stars—its, rather than of
- Done
- (described below Organic compounds section). —I don't like this. I'd put in a note, or at least put the section title in quotes
- Note made. Yeah, I am sort of threading a needle (we have been back and forth on having CF4 in this section).
- oxygen is at oxidation state +2) —perhaps formal oxidation state? We know it's not really ionic
- Good. Done.
- The discovery was touted—touted seem too informal
- Reworded and made active voice.
- Streptomyces cattleya—italics for binomial
- King Goblin? (I'm scared to go in.)
Thanks. Keep the pass through going! :-) 98.117.75.177 (talk) 16:21, 13 October 2013 (UTC)
Nergaal
[edit]- You should use some {{quote}} template for the Nobel citation. Nergaal (talk) 16:15, 8 October 2013 (UTC)
- Added one. If it can be made better, please help us, this techie stuff hard on me.
- For the sake of structure, CF4 should be discussed/mentioned in the nonmetal fluorides section. Nergaal (talk) 16:37, 8 October 2013 (UTC)
- Added something. (Please note, we've had it before and been asked to rip it out...starting to get on the merry go round. Please tweak the wording if you want.)
-Please carry on. Seeing you go through the thing and appreciate the front to back work.108.162.44.194 (talk) 23:09, 8 October 2013 (UTC)
-The Barret 1967 paper covers the details of the violent phase transition (click DOI and see last sentence of abstract...is more in the paper itself). Ref was one sentence down (now duplicated) to both sentences. Need someone to fix the formatting glitch (fn numbering).
- I removed it because it seems to be a case of TMI. Having a note there might work better and be less distracting to a casual reader.
- OK. I put it in a note.
- I removed it because it seems to be a case of TMI. Having a note there might work better and be less distracting to a casual reader.
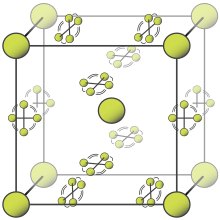
- I am pretty sure that the bottom animation has the wrong caption. Nergaal (talk) 12:10, 11 October 2013 (UTC)
- Added a citation for the caption.
Hawkeye7
[edit]What a great article. Very impressive. I have read through the historical and industrial sections. My only quibble is with this bit:
Karl O. Christe discovered a purely chemical preparation of fluorine gas. However, he stated in his work that the basics were known 50 years before the actual reaction. The main idea is that some metal fluoride anions do not have a neutral counterpart (or those are very unstable) and their acidifying would result in chemical oxidation, rather than formation of the expected molecules. Christe lists the following reactions as a possible way:
There are two awkward bits here: "or those are very unstable" and "Christe lists the following reactions as a possible way". For the first I would suggest "or have ones that are very unstable" - the grammatical awkwardness being about "those". The second changes tense from past to future, and the use of "possible" makes it sound at first reading that he suggested rather than discovered it. I suggest something in the active like like "Christie's process was:"
But these are just quibbles. I think that it is really good. Hawkeye7 (talk) 20:12, 8 October 2013 (UTC)
- Picked me up big time with the compliment. Aussie Aussie oi, oi. Made the changes, tweak more if needed.108.162.44.194 (talk) 23:23, 8 October 2013 (UTC)
Casliber
[edit]- Agree with using atorvastatin and fluoxetine in the article - the trade names are receding in popularity as generic versions multiply anyway. Cas Liber (talk · contribs) 05:03, 10 October 2013 (UTC)
- I think current state of readers is what matters and many more recognize the 3 brand names than the generic names, but I met you halfway and went the parenthetical route.
- This phase is transparent and soft with significant disorder of the molecules. - dunno what that means (but I can guess) - link or explain?
- I assume disorder is your sticking point. We don't have a good blue link for this and I can't do an easy quick explanation. The way for me to really handle this is more slickly with a picture of the xtal structure. (perhaps a diptych of the two phases). Is a very nice nice diagram of the disordered cubic phase in one of the science papers (I think Pauling). Shows molecules rotating (better than the KCN diagram below from Wiki). I need some significant help in getting that diagram sketched (or an equivalent, not sure if a rights issue for us to just recreate it...as it is diagrammatic) as well as just copy of the article (I don't hold it any more). I have tried in the past but not gotten it done. Either people said it was too hard or Matsci gave it a try but I don't think he had the right paper for the diagram and his image really did not look good.
- Actually I thought that crystallographic disorder is a notion that many users will probably not know. I tried to find an appropriate wikilink but failed. I strongly suggest to have some sort of wikilink for those outside of chemistry (although I bet there are many organic chemists that will probably not know either). Nergaal (talk) 12:14, 11 October 2013 (UTC)
- Someone start a stub, then. We all think one is needed. I gave a reference to a review (in note). We can build a para long explanation pretty easily. could get lots of incoming links from articles like KCN.71.127.137.171 (talk) 19:38, 12 October 2013 (UTC)
- Actually I thought that crystallographic disorder is a notion that many users will probably not know. I tried to find an appropriate wikilink but failed. I strongly suggest to have some sort of wikilink for those outside of chemistry (although I bet there are many organic chemists that will probably not know either). Nergaal (talk) 12:14, 11 October 2013 (UTC)
There should be some folks willing to help with this (can't remember who now as I've not buffed chemical articles meself...) - need to think/jog memory...Cas Liber (talk · contribs) 10:10, 11 October 2013 (UTC)
- Added an explanatory footnote--if we got a diagram, Cas, not even sure if we would show it in this article. I had a centered table of alpha fluorine and the phase diagram earlier, but had cut THAT, based on desire for lower image/text ratio from another critic. If I had the picture would definitely use it in the spinout article, though. See here for an earlier attempt to get the diagram made. MatSci made an attempt for me too (see Commons), but he didn't do it right...it lacks the illustrative impact of the 1970 Pauling diagram.108.162.44.194 (talk) 11:31, 11 October 2013 (UTC)
- Link to diagram of beta-fluorine: [6]. 98.117.75.177 (talk) 15:42, 13 October 2013 (UTC)
Axl
[edit]From the lead section, paragraph 2: "In the universe, fluorine is rare, for a light element." Why not "for an element" rather than "for a light element"? Axl ¤ [Talk] 09:51, 13 October 2013 (UTC)- Because it is not rare, for "an element", but only "for a light element". In general, light elements (say through iron) are more common than heavy elements in the universe because they are formed in normal nuclear fusion of burning stars rather than in rare nova explosions. See discussion in article body. If it's not good enough, than I can add some explanatory note or work on the text with a sentence or two of explanation: "In general, elements are more common the lighter they are...blabla..."71.127.137.171 (talk) 14:18, 13 October 2013 (UTC)
- I understand your argument, but this depends on the meanings of "light" and "rare". You have now defined the "light" elements as through to iron. (I assume that "light" is an acceptable shorthand for "low atomic mass".) The table in "Origin and occurrence" shows that fluorine is indeed rarer than its neighbours on the periodic table. But at what level is an element classified as "rare" in general? Axl ¤ [Talk] 18:12, 13 October 2013 (UTC)
Sources make an observation of relative rarity, Axl. I didn't invent the observation. It's not some...digital threshold, but also a true, pertinent, commonly noted insight. Can check the sources in article (one is online) and here are more:
An Element Apart...For one thing its rare....It's like a shack stuck among mansions.
[Actually just rephrase it. Something is making you uncomfortable, so just change it. ;-)]
71.127.137.171 (talk) 19:00, 13 October 2013 (UTC)
- Well, I would rather that we reach an agreement, perhaps a compromise. How about "In the universe, fluorine is a relatively rare element... "? Axl ¤ [Talk] 21:23, 13 October 2013 (UTC)
- I can live with that. For one thing, it's a more direct construction. Lower in body, we can have the details of the caveat. (done)
- I have simplified it even more in lead to just the ranking. In body I added some more explanation and a reference. Whole kerfuffle has been additive.71.127.137.171 (talk) 22:20, 13 October 2013 (UTC)
- Thanks. Axl ¤ [Talk] 23:20, 13 October 2013 (UTC)
- I have simplified it even more in lead to just the ranking. In body I added some more explanation and a reference. Whole kerfuffle has been additive.71.127.137.171 (talk) 22:20, 13 October 2013 (UTC)
From the lead section, paragraph 2: "The other half is converted to hydrogen fluoride, a dangerous acid which is the precursor to most synthetic fluorochemicals." Surely the "danger" depends on the concentration and on how it is handled? Perhaps "strong" rather than "dangerous"? Axl ¤ [Talk] 18:31, 13 October 2013 (UTC)
No. It is NOT a chemically "strong" acid. It is chemically "weak". But it is much more dangerous than the strong chemical acids. Please read the section on the burns. A British man had a small burn and had to have his leg cut off and then died anyway (even after taking immediate actions). A huge amount of 19th century chemists died or were poisoned or blinded (see note in history). I'm not over-egging the pudding. "Dangerous" is a simple one word descriptor for HF. By using it, I am able to have the lead summarize a subsection of the article. (Of course proper handling avoids injury, but that's a consequence of the danger, not a change to it.) It really is nasty stuff. I will slop around concentrated nitric, hydrochloric, sulfuric in a heartbeat. Think nothing of a jar of aqua regia in an open container by the sink that could get nocked over. But HF? Treat with respect.
I mean, it's not anthrax bacteria. You can use it...and it is used in industry and labs. I was in a lab group that used it a fair amount. But still we had two major accidents where we almost had a very serious injury. You can use it, use it, use it...but then that one time you get it on you...bad news. I definitely am more scared of the bottle with HF on it than I am of the concentrated mineral acids or ceramic temperature ovens.
71.127.137.171 (talk) 19:00, 13 October 2013 (UTC)
- Thank you, my mistake. It has been many years since I studied A-level chemistry. A little knowledge is a dangerous thing. Axl ¤ [Talk] 21:27, 13 October 2013 (UTC)
- No sweat, I don't mean to bludgeon you. I come armed with huge knowledge after playing with this article so long. No problem, with skepticism and intuitive concerns. It's how I engage also.
In the lead section, paragraph 3, we have "aluminium" [British spelling]. In "Characteristics", subsection "Atomic structure", we have "picometers" [US spelling]. Axl ¤ [Talk] 23:10, 13 October 2013 (UTC)
The article itself is in American English (just its history as well as confort factor of the two main authors). For element names, there is a negotiated settlement across all element articles (maybe even all chemicals articles), to use the IUPAC names. So the Brits get aluminum and caesium and the Americans get sulfur. If you want, I can dig up the talk pages and policy and all that from the Wikiprojects. 's true though. ;-)
71.127.137.171 (talk) 23:43, 13 October 2013 (UTC)
- "So the Brits get aluminum." Don't you mean "aluminium"? ;-) Axl ¤ [Talk] 23:45, 13 October 2013 (UTC)
- Overpaid, oversexed and over here. ;-) 71.127.137.171 (talk) 00:00, 14 October 2013 (UTC)
From "Occurrence", subsection "Universe", paragraph 1: "One science writer described fluorine as a "shack amongst mansions" in terms of abundance." That's a stupid analogy. The difference between shacks and mansions is not just size. Is the author implying that the build quality of fluorine is lower than that of the other elements? Given that the preceding two sentences already describe fluorine's scarcity, this sentence adds nothing. Axl ¤ [Talk] 08:33, 14 October 2013 (UTC)
- I have deleted the sentence. Axl ¤ [Talk] 14:58, 14 October 2013 (UTC)
- From "Occurrence", subsection "Universe", paragraph 1: "Any fluorine created within stars is rapidly eliminated through strong nuclear fusion reactions." What is the meaning of "strong" in this context? Axl ¤ [Talk] 15:00, 14 October 2013 (UTC)
- I guess it means likely to uccur because of cross sections and avaialbalbe reactants...but then rapid sort of already covers that. I tried to reword and just cut the strong. Please take a look at ref 15 and see if you want to tweak the explication. Basically little fluorine gets made cause the buildup reactions just sort of skip it. And the what does get made is zapped readily.
- I don't really like the addition of the new sentence, especially the abbreviation, but I suppose that the meaning is clear. Axl ¤ [Talk] 10:01, 15 October 2013 (UTC)
- The point is that there are two reasons why so little F. (1) normal reactions don't make it, (2) what does get made is prey to immediate reactions with H, He (very common in stars) to zap it away. However, we say that is fine as long as reader understands. I got the impression there was confusion, so I spelled it out more. If you want to rewrite, please do.208.44.87.91 (talk) 10:58, 15 October 2013 (UTC)
- I don't really like the addition of the new sentence, especially the abbreviation, but I suppose that the meaning is clear. Axl ¤ [Talk] 10:01, 15 October 2013 (UTC)
From "Occurrence", subsection "Universe", paragraph 2: "In Wolf-Rayet stars (blue stars over 40 times heavier than the Sun)." Shouldn't "Wolf-Rayet" use an en dash rather than a hyphen? Axl ¤ [Talk] 15:13, 14 October 2013 (UTC)- sorry, my laptop was missing that key. ;-) Fixed.
[Please carry on, doc. I see you going through it in detail and appreciate it. I hope it is a little fun/interesting to you, too.208.44.87.91 (talk) 23:58, 14 October 2013 (UTC)]
Would it be reasonable to explain why fluorine is more common on Earth than it is in the universe in general, or is that beyond the scope of this article? Axl ¤ [Talk] 10:04, 15 October 2013 (UTC)- Probably. I would need to research and add new references. I believe the relative concentration has to do with compound (i.e. mineral) forming tendencies. Why there is more F than Ne on Earth for instance. It could also be interesting to compare to crustal oxygen.208.44.87.91 (talk) 11:15, 15 October 2013 (UTC)
- I don't think I can get this done. I looked at a few references but was not finding enough to really talk about concentration of fluorine. Undoubtedly has to do with rocky planet formation and compound forming habits (versus volatiles). But I just can't find the sources to really explain it that way. If you can get someone to do it, great. I give up.208.44.87.91 (talk) 00:48, 18 October 2013 (UTC)
- Done, added explanation and reffed it.38.107.128.2 (talk) 00:37, 19 October 2013 (UTC)
- I don't think I can get this done. I looked at a few references but was not finding enough to really talk about concentration of fluorine. Undoubtedly has to do with rocky planet formation and compound forming habits (versus volatiles). But I just can't find the sources to really explain it that way. If you can get someone to do it, great. I give up.208.44.87.91 (talk) 00:48, 18 October 2013 (UTC)
- Probably. I would need to research and add new references. I believe the relative concentration has to do with compound (i.e. mineral) forming tendencies. Why there is more F than Ne on Earth for instance. It could also be interesting to compare to crustal oxygen.208.44.87.91 (talk) 11:15, 15 October 2013 (UTC)
- Well, there is an implication but not a clear explanation. Anyway, this isn't so important for the article. Axl ¤ [Talk] 08:21, 21 October 2013 (UTC)
The lead section, paragraph 3, states that cryolite is a "manmade inorganic fluoride". However "Occurrence", subsection "Earth", implies that cryolite does occur naturally. Axl ¤ [Talk] 10:37, 15 October 2013 (UTC)- Until the mid-80s, the natural sources were the commercial sources. Now, the main mine is depleted and industry relies on synthesized cryolite.
- The current text is better. Axl ¤ [Talk] 09:33, 17 October 2013 (UTC)
From "Occurrence", subsection "Earth": "the alkaline earth fluorides precipitate out of water." What are "alkaline earth fluorides"? Axl ¤ [Talk] 09:39, 17 October 2013 (UTC)- Explained this.
- Thanks. Axl ¤ [Talk] 08:35, 21 October 2013 (UTC)
From "Occurrence", subsection "Earth", last paragraph: "One form of fluorite, antozonite, has an ozone smell (suggestive of fluorine) when crushed." The statement implies that fluorine smells similar to ozone. However the article "Antozonite" states that ozone is formed by chemical reaction when the fluorine is released into the air. Axl ¤ [Talk] 10:25, 17 October 2013 (UTC)- Simplified to "suggestive of fluorine". There are some sources that say it smells like fluorine, but also a 1937 source that says it smells like fluorine's reaction products (HF, O3). (which opens a whole can of worms on what just released fluorine smells like). Added the more recent ref that just says fluorine smell. I think as stated now is simpler story and the "suggestive" is enough of a caveat.208.44.87.91 (talk) 12:01, 17 October 2013 (UTC)
- Okay. Axl ¤ [Talk] 08:42, 21 October 2013 (UTC)
- In "History", subsection "Later developments", I am not convinced that the information about CFCs, Freon & PTFE are directly relevant. However this isn't a major point. Axl ¤ [Talk] 11:16, 30 October 2013 (UTC)
- I cut one para in response to your comment (1970s CFC concerns). Rest, I think belongs there. Teflon story in particular is commonly included in popular histories of fluorine. Every element is different, but this one a lot of it's story is around compounds and the development of the fluorochemical industry.71.127.137.171 (talk) 23:59, 1 November 2013 (UTC)
Infobox help
[edit]Could someone please fix the issue Wehwalt raised with the reference12 at bottom of infobox? And the fact/unit problem (see article talk)? I can't edit the template. the gnome-owner of the template is pissed at me since he has been bloating the thing for the last while and I want it tightened up...but just ignore that kerfuffle and make the two simple fixes, please.208.44.87.91 (talk) 00:59, 23 October 2013 (UTC)