 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
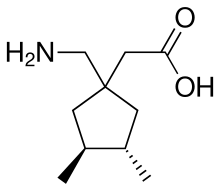
| Formula | C10H19NO2 |
| Molar mass | 185.267 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Atagabalin (PD-0200,390) is a drug developed by Pfizer and related to gabapentin, which similarly binds to the α2δ calcium channels (1 and 2).[1] It was under development as a treatment for insomnia,[2][3][4] but was discontinued following unsatisfactory trial results.
See also
[edit]References
[edit]- ^ Blakemore DC, Bryans JS, Carnell P, Carr CL, Chessum NE, Field MJ, Kinsella N, Osborne SA, Warren AN, Williams SC (January 2010). "Synthesis and in vivo evaluation of bicyclic gababutins". Bioorganic & Medicinal Chemistry Letters. 20 (2): 461–4. doi:10.1016/j.bmcl.2009.11.118. PMID 20005103.
- ^ Corrigan B, Feltner DE, Ouellet D, Werth JL, Moton AE, Gibson G (August 2009). "Effect of renal impairment on the pharmacokinetics of PD 0200390, a novel ligand for the voltage-gated calcium channel alpha-2-delta subunit". British Journal of Clinical Pharmacology. 68 (2): 174–80. doi:10.1111/j.1365-2125.2009.03444.x. PMC 2767279. PMID 19694735.
- ^ Quintero JE, Pomerleau F, Huettl P, Johnson KW, Offord J, Gerhardt GA (May 2011). "Methodology for rapid measures of glutamate release in rat brain slices using ceramic-based microelectrode arrays: Basic characterization and drug pharmacology". Brain Research. 1401: 1–9. doi:10.1016/j.brainres.2011.05.025. PMC 3197737. PMID 21664606.
- ^ Kjellsson MC, Ouellet D, Corrigan B, Karlsson MO (June 2011). "Modeling Sleep Data for a New Drug in Development using Markov Mixed-Effects Models". Pharmaceutical Research. 28 (10): 2610–27. doi:10.1007/s11095-011-0490-x. PMID 21681607. S2CID 22241527.