 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601020 |
| Routes of administration | IV only |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% (as IV bolus, infusion) |
| Protein binding | 70 to 80% |
| Metabolism | Liver (12%) |
| Elimination half-life | 2.3 hours (mean, in CHF) |
| Excretion | Urine (85% as unchanged drug) within 24 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.071.709 |
| Chemical and physical data | |
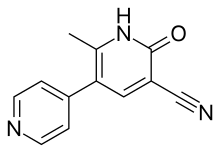
| Formula | C12H9N3O |
| Molar mass | 211.224 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.344 g/cm3 |
| Melting point | 315 °C (599 °F) |
| |
| |
| (verify) | |
Milrinone, sold under the brand name Primacor, is a pulmonary vasodilator[2] used in patients who have heart failure. It is a phosphodiesterase 3 inhibitor that works to increase the heart's contractility and decrease pulmonary vascular resistance. Milrinone also works to vasodilate which helps alleviate increased pressures (afterload) on the heart, thus improving its pumping action. While it has been used in people with heart failure for many years, studies suggest that milrinone may exhibit some negative side effects that have caused some debate about its use clinically.[3][4]
Overall, milrinone supports ventricular functioning of the heart by decreasing the degradation of cyclic adenosine monophosphate (cAMP) and thus increasing phosphorylation levels of many components in the heart that contribute to contractility and heart rate. Milrinone is used as a drug that causes positive inotropy and it will lead to an increased force of contraction. Milrinone use following cardiac surgery has been under some debate because of the potential increase risk of postoperative atrial arrhythmias.[5] However, in the short term milrinone has been deemed beneficial to those experiencing heart failure and an effective therapy to maintain heart function following cardiac surgeries. There is no evidence of any long term beneficial effects on survival.[6] In critically ill patients with evidence of cardiac dysfunction there is limited good quality evidence to recommend its use.[7]
Milrinone is administered IV only and eliminated unchanged in the urine. Dose adjustment is required for patients with renal impairment.[8]
Contractility in the heart
[edit]People experiencing some forms of heart failure have a significant decrease in the contractile ability of muscle cells in the heart (cardiomyocytes).[9] This impaired contractility occurs through a number of mechanisms. Some of the main problems associated with decreased contractility in those with heart failure are issues arising from imbalances in the concentration of calcium.[10] Calcium permits myosin and actin to interact which allows initiation of contraction within the cardiomyocytes. In those with heart failure there may be a decreased amount of calcium within the cardiomyocytes reducing the available calcium to initiate contraction.[11] When contractility is decreased the amount of blood being pumped out of the heart into circulation is decreased as well. This reduction in cardiac output can cause many systemic implications such as fatigue, syncope and other issues associated with decreased blood flow to peripheral tissues.[12]
Mechanism of action
[edit]Milrinone is a phosphodiesterase-3 inhibitor. This drug inhibits the action of phosphodiesterase-3 and thus prevents degradation of cAMP. Normally, cAMP causes increased activation of protein kinase A (PKA). PKA is an enzyme that phosphorylates many elements of the contractile machinery within the heart cell. In the short term this leads to an increased force of contraction. Phosphodiesterases are enzymes responsible for the breakdown of cAMP. Therefore, when phosphodiesterases lower the level of cAMP in the cell they also lower the active fraction of PKA within the cell and reduce the force of contraction.[13]
With increased cAMP levels there is an increase in the activation of PKA. This PKA will phosphorylate many components of the cardiomyocyte such as calcium channels and components of the myofilaments. Phosphorylation of calcium channels permits an increase in calcium influx into the cell. This increase in calcium influx results in increased contractility. PKA also phosphorylates potassium channels promoting their action. Potassium channels are responsible for repolarization of the cardiomyocytes therefore increasing the rate at which cells can depolarize and generate contraction. PKA also phosphorylates components on myofilaments allowing actin and myosin to interact more easily and thus increasing contractility and the inotropic state of the heart. Milrinone allows stimulation of cardiac function independently of β-adrenergic receptors which appear to be down-regulated in those with heart failure.[13]
Clinical use
[edit]Milrinone is a commonly used therapy for severe pulmonary arterial hypertension (PAH),[14] often in combination with other medications such as sildenafil.[15] Targeting PDE3 with optimal doses and timing, milrinone prevents allergic inflammation in HDM-driven models of allergic airway inflammation.[16]
It can be used in cardiopulmonary bypass cases, as it increases the flow in saphenous grafts and has a beneficiary effect in left ventricle function.[17]
Adverse effects
[edit]Common adverse effects include ventricular arrhythmias (including ventricular ectopy and nonsustained ventricular tachycardia), supraventricular arrhythmias, hypotension, and headache.[18]
Synthesis
[edit]
The reaction between 4-methylpyridine and methyl acetate gives 4-pyridyl acetone (4-acetonylpyridine) [6304-16-1] (1). The Knoevenagel condensation type reaction between this and DMF-dimethylacetal [4637-24-5] (2) affords CID:3018775 (3). Then base catalyzed reaction of this with cyanoacetamide (4) completes the synthesis of milrinone (5).
References
[edit]- ^ "Active substance: milrinone" (PDF). List of nationally authorised medicinal products. European Medicines Agency. 10 June 2022.
- ^ Baxter FJ, Whippey A (November 2020). "Amniotic Fluid Embolism Treated With Inhaled Milrinone: A Case Report". A&A Practice. 14 (13): e01342. doi:10.1213/XAA.0000000000001342. PMID 33185413. S2CID 226851766.
- ^ Packer M (December 1990). "Calcium channel blockers in chronic heart failure. The risks of "physiologically rational" therapy". Circulation. 82 (6): 2254–2257. doi:10.1161/01.cir.82.6.2254. PMID 2242549. S2CID 11255642.
- ^ Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, et al. (November 1991). "Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group". The New England Journal of Medicine. 325 (21): 1468–1475. doi:10.1056/NEJM199111213252103. PMID 1944425.
- ^ Fleming GA, Murray KT, Yu C, Byrne JG, Greelish JP, Petracek MR, et al. (October 2008). "Milrinone use is associated with postoperative atrial fibrillation after cardiac surgery". Circulation. 118 (16): 1619–1625. doi:10.1161/CIRCULATIONAHA.108.790162. PMC 2770257. PMID 18824641.
- ^ British National Formulary (66th ed.). London: BMJ Group and Pharmaceutical Press. September 2013.
- ^ Koster G, Bekema HJ, Wetterslev J, Gluud C, Keus F, van der Horst IC (September 2016). "Milrinone for cardiac dysfunction in critically ill adult patients: a systematic review of randomised clinical trials with meta-analysis and trial sequential analysis". Intensive Care Medicine. 42 (9): 1322–1335. doi:10.1007/s00134-016-4449-6. PMC 4992029. PMID 27448246.
- ^ "Milrinone Dosage Guide + Max Dose, Adjustments". Drugs.com. Retrieved 2023-01-03.
- ^ de Tombe PP (February 1998). "Altered contractile function in heart failure". Cardiovascular Research. 37 (2): 367–380. doi:10.1016/s0008-6363(97)00275-7. PMID 9614494.
- ^ Ward ML, Crossman DJ (July 2014). "Mechanisms underlying the impaired contractility of diabetic cardiomyopathy". World Journal of Cardiology. 6 (7): 577–584. doi:10.4330/wjc.v6.i7.577. PMC 4110606. PMID 25068018.
- ^ Szent-Györgyi AG (July 1975). "Calcium regulation of muscle contraction". Biophysical Journal. 15 (7): 707–723. Bibcode:1975BpJ....15..707S. doi:10.1016/S0006-3495(75)85849-8. PMC 1334730. PMID 806311.
- ^ King J, Lowery DR (2022). "Physiology, Cardiac Output". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 29262215.
- ^ a b Zimmerman J, Cahalan M (January 2013). "Chapter 22 - Vasopressors and Inotropes". In Hemmings HC, Egan TD (eds.). Pharmacology and Physiology for Anesthesia. Philadelphia: W.B. Saunders. pp. 390–404. doi:10.1016/B978-1-4377-1679-5.00022-3. ISBN 978-1-4377-1679-5. S2CID 79282649. Retrieved 2023-03-14.
- ^ McNamara PJ, Shivananda SP, Sahni M, Freeman D, Taddio A (January 2013). "Pharmacology of milrinone in neonates with persistent pulmonary hypertension of the newborn and suboptimal response to inhaled nitric oxide". Pediatric Critical Care Medicine. 14 (1): 74–84. doi:10.1097/PCC.0b013e31824ea2cd. PMID 23132395. S2CID 7696208.
- ^ Hui-li G (June 2011). "The management of acute pulmonary arterial hypertension". Cardiovascular Therapeutics. 29 (3): 153–175. doi:10.1111/j.1755-5922.2009.00095.x. PMID 20560976.
- ^ Beute J, Lukkes M, Koekoek EP, Nastiti H, Ganesh K, de Bruijn MJ, et al. (January 2018). "A pathophysiological role of PDE3 in allergic airway inflammation". JCI Insight. 3 (2). doi:10.1172/jci.insight.94888. PMC 5821178. PMID 29367458.
- ^ Arbeus M, Axelsson B, Friberg O, Magnuson A, Bodin L, Hultman J (February 2009). "Milrinone increases flow in coronary artery bypass grafts after cardiopulmonary bypass: a prospective, randomized, double-blind, placebo-controlled study". Journal of Cardiothoracic and Vascular Anesthesia. 23 (1). Elsevier BV: 48–53. doi:10.1053/j.jvca.2008.07.005. PMID 18834820.
- ^ "Milrinone Lactate Monograph". drugs.com.
- ^ BE886336 idem G. Y. Lesher, R. E. Philion, U.S. patent 4,313,951 (1982 both to Sterling).
- ^ US 4264603, Lesher GY, Gruett MD, issued 1981, assigned to Sterling Drug Inc.
- ^ US 4413127, Singh B, issued 1983, assigned to Sterling
- ^ Singh B (1985). "A Novel Synthesis of 1,6-Dihydro-2-methyl-6-oxo[3,4'-bipyridine]-5-carbonitrile (Milrinone)". Heterocycles. 23 (6): 1479. doi:10.3987/R-1985-06-1479.
- ^ Shiao MJ, Shyu LM, Chen CF (1990). "Synthesis of Milrinone, a Cardiotonic Agent". Heterocycles. 31 (3): 523. doi:10.3987/COM-89-5276.
- ^ CN 104326975, Yan H, Deng A, "Preparation method of high-purity milrinone", issued 2015, assigned to ZHENGZHOU SIHUAN MEDICINE ARTICLE Co Ltd
- ^ CN 104387320, Ao L, Zhang B, Pan J, Chen Y, "Preparation Method for High-Purity Milrinone", issued 2018, assigned to Huzhou Zhanwang Pharmaceutical Co., Ltd.
- ^ CN 103848779, Tan X, Jing Y, Wang F, Liu H, Yu U, "Preparation method of 1-(4-pyridyl) acetone", issued 2014, assigned to University of Jinan