| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.035.935 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| VI2 | |
| Appearance | black mica-like |
| Density | 5.44 g/cm3 |
| Related compounds | |
Other anions
|
vanadium(II) chloride, vanadium(II) bromide |
Related compounds
|
vanadium(III) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
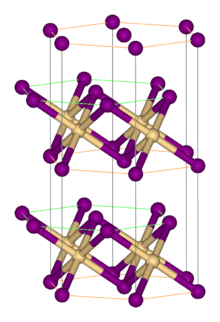
Vanadium(II) iodide is the inorganic compound with the formula VI2. It is a black micaceous solid. It adopts the cadmium iodide structure, featuring octahedral V(II) centers.[1] The hexahydrate [V(H2O)6]I2, an aquo complex, is also known. It forms red-violet crystals. The hexahydrate dehydrates under vacuum to give a red-brown tetrahydrate with the formula V(H2O)4I2.[2]
Preparation
[edit]The original synthesis of VI2 involved reaction of the elements.[1]
Solvated vanadium(II) iodides can be prepared by reduction of vanadium(III) chlorides with trimethylsilyl iodide.[3] It reacts with anhydrous ammonia to give the hexaammine complex.[4]
References
[edit]- ^ a b Klemm, Wilhelm; Grimm, Ludwig (1942). "Zur Kenntnis der Dihalogenide des Titans und Vanadins". Zeitschrift für Anorganische und Allgemeine Chemie. 249 (2): 198–208. doi:10.1002/zaac.19422490204.
- ^ Seifert, Hans-Joachim; Gerstenberg, Burkhard (1962). "Darstellung von Vanadin(II)-Verbindungen aus wäßriger Lösung". Zeitschrift für Anorganische und Allgemeine Chemie. 315 (1–2): 56–63. doi:10.1002/zaac.19623150108.
- ^ Hitchcock, Peter B.; Hughes, David L.; Leigh, G. Jeffery; Sanders, J. Roger; De Souza, Jaisa; McGarry, Celine J.; Larkworthy, Leslie F. (1994). "Preparation of New Vanadium(II) Iodides and Crystal Structure of Hexakis(acetonitrile)vanadium(II)(Tetraiodide)". Journal of the Chemical Society, Dalton Transactions (24): 3683. doi:10.1039/DT9940003683.
- ^ Eßmann, Ralf; Kreiner, Guido; Niemann, Anke; Rechenbach, Dirk; Schmieding, Axel; Sichla, Thomas; Zachwieja, Uwe; Jacobs, Herbert (1996). "Isotype Strukturen einiger Hexaamminmetall(II)-halogenide von 3d-Metallen: V(NH3)6I2, Cr(NH3)6I2, Mn(NH3)6Cl2, Fe(NH3)6Cl2, Fe(NH3)6Br2, Co(NH3)6Br2 und Ni(NH3)6Cl2". Zeitschrift für Anorganische und Allgemeine Chemie. 622 (7): 1161–1166. doi:10.1002/zaac.19966220709.