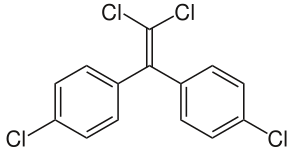
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,1′-(2,2,2-Trichloroethane-1,1-diyl)bis(4-chlorobenzene) | |||
| Other names
Dichlorodiphenyltrichloroethane (DDT)
Clofenotane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.023 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C14H9Cl5 | |||
| Molar mass | 354.48 g·mol−1 | ||
| Density | 0.99 g/cm3 | ||
| Melting point | 108.5 °C (227.3 °F; 381.6 K) | ||
| Boiling point | 260 °C (500 °F; 533 K) (decomposes) | ||
| 25 μg/L (25 °C)[1] | |||
| Pharmacology | |||
| QP53AB01 (WHO) | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Toxic, dangerous to the environment, suspected carcinogen | ||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H301, H350, H372, H410 | |||
| P201, P202, P260, P264, P270, P273, P281, P301+P310, P308+P313, P314, P321, P330, P391, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 72–77 °C; 162–171 °F; 345–350 K[3] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
113–800 mg/kg (rat, oral)[1] 250 mg/kg (rabbit, oral) 135 mg/kg (mouse, oral) 150 mg/kg (guinea pig, oral)[2] | ||
| NIOSH (US health exposure limits):[4] | |||
PEL (Permissible)
|
TWA 1 mg/m3 [skin] | ||
REL (Recommended)
|
Ca TWA 0.5 mg/m3 | ||
IDLH (Immediate danger)
|
500 mg/m3 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Dichlorodiphenyltrichloroethane, commonly known as DDT, is a colorless, tasteless, and almost odorless crystalline chemical compound,[5] an organochloride. Originally developed as an insecticide, it became infamous for its environmental impacts. DDT was first synthesized in 1874 by the Austrian chemist Othmar Zeidler. DDT's insecticidal action was discovered by the Swiss chemist Paul Hermann Müller in 1939. DDT was used in the second half of World War II to limit the spread of the insect-borne diseases malaria and typhus among civilians and troops. Müller was awarded the Nobel Prize in Physiology or Medicine in 1948 "for his discovery of the high efficiency of DDT as a contact poison against several arthropods".[6] The WHO's anti-malaria campaign of the 1950s and 1960s relied heavily on DDT and the results were promising, though there was a resurgence in developing countries afterwards.[7][8]
By October 1945, DDT was available for public sale in the United States. Although it was promoted by government and industry for use as an agricultural and household pesticide, there were also concerns about its use from the beginning.[9] Opposition to DDT was focused by the 1962 publication of Rachel Carson's book Silent Spring. It talked about environmental impacts that correlated with the widespread use of DDT in agriculture in the United States, and it questioned the logic of broadcasting potentially dangerous chemicals into the environment with little prior investigation of their environmental and health effects. The book cited claims that DDT and other pesticides caused cancer and that their agricultural use was a threat to wildlife, particularly birds. Although Carson never directly called for an outright ban on the use of DDT, its publication was a seminal event for the environmental movement and resulted in a large public outcry that eventually led, in 1972, to a ban on DDT's agricultural use in the United States.[10] Along with the passage of the Endangered Species Act, the United States ban on DDT is a major factor in the comeback of the bald eagle (the national bird of the United States) and the peregrine falcon from near-extinction in the contiguous United States.[11][12]
The evolution of DDT resistance and the harm both to humans and the environment led many governments to curtail DDT use.[13] A worldwide ban on agricultural use was formalized under the Stockholm Convention on Persistent Organic Pollutants, which has been in effect since 2004. Recognizing that total elimination in many malaria-prone countries is currently unfeasible in the absence of affordable/effective alternatives for disease control, the convention exempts public health use within World Health Organization (WHO) guidelines from the ban.[14]
DDT still has limited use in disease vector control because of its effectiveness in killing mosquitos and thus reducing malarial infections, but that use is controversial due to environmental and health concerns.[15][16] DDT is one of many tools to fight malaria, which remains the primary public health challenge in many countries. WHO guidelines require that absence of DDT resistance must be confirmed before using it.[17] Resistance is largely due to agricultural use, in much greater quantities than required for disease prevention.[17]
Properties and chemistry
[edit]DDT is similar in structure to the insecticide methoxychlor and the acaricide dicofol. It is highly hydrophobic and nearly insoluble in water but has good solubility in most organic solvents, fats and oils. DDT does not occur naturally and is synthesised by consecutive Friedel–Crafts reactions between chloral (CCl
3CHO) and two equivalents of chlorobenzene (C
6H
5Cl), in the presence of an acidic catalyst.[1] DDT has been marketed under trade names including Anofex, Cezarex, Chlorophenothane, Dicophane, Dinocide, Gesarol, Guesapon, Guesarol, Gyron, Ixodex, Neocid, Neocidol and Zerdane; INN is clofenotane.[5]
Isomers and related compounds
[edit]Commercial DDT is a mixture of several closely related compounds. Due to the nature of the chemical reaction used to synthesize DDT, several combinations of ortho and para arene substitution patterns are formed. The major component (77%) is the desired p,p' isomer. The o,p' isomeric impurity is also present in significant amounts (15%). Dichlorodiphenyldichloroethylene (DDE) and dichlorodiphenyldichloroethane (DDD) make up the balance of impurities in commercial samples. DDE and DDD are also the major metabolites and environmental breakdown products.[5] DDT, DDE and DDD are sometimes referred to collectively as DDX.[18]
- Components of commercial DDT
-
p,p'-DDT
(desired compound) -
o,p'-DDT
(isomeric impurity) -
p,p'-DDE
(impurity) -
p,p'-DDD
(impurity)
Production and use
[edit]DDT has been formulated in multiple forms, including solutions in xylene or petroleum distillates, emulsifiable concentrates, water-wettable powders, granules, aerosols, smoke candles and charges for vaporizers and lotions.[19]
From 1950 to 1980, DDT was extensively used in agriculture – more than 40,000 tonnes each year worldwide[20] – and it has been estimated that a total of 1.8 million tonnes have been produced globally since the 1940s.[1] In the United States, it was manufactured by some 15 companies, including Monsanto, Ciba,[21] Montrose Chemical Company, Pennwalt,[22] and Velsicol Chemical Corporation.[23] Production peaked in 1963 at 82,000 tonnes per year.[5] More than 600,000 tonnes (1.35 billion pounds) were applied in the US before the 1972 ban. Usage peaked in 1959 at about 36,000 tonnes.[24]
China ceased production in 2007,[25] leaving India the only country still manufacturing DDT; it is the largest consumer.[7] In 2009, 3,314 tonnes were produced for malaria control and visceral leishmaniasis. In recent years, in addition to India, just seven other countries, all in Africa, are still using DDT.[26]
Mechanism of insecticide action
[edit]In insects, DDT opens voltage-sensitive sodium ion channels in neurons, causing them to fire spontaneously, which leads to spasms and eventual death.[27] Insects with certain mutations in their sodium channel gene are resistant to DDT and similar insecticides.[27] DDT resistance is also conferred by up-regulation of genes expressing cytochrome P450 in some insect species,[28] as greater quantities of some enzymes of this group accelerate the toxin's metabolism into inactive metabolites. Genomic studies in the model genetic organism Drosophila melanogaster revealed that high level DDT resistance is polygenic, involving multiple resistance mechanisms.[29] In the absence of genetic adaptation, Roberts and Andre 1994 find behavioral avoidance nonetheless provides insects with some protection against DDT.[30] The M918T mutation event produces dramatic kdr for pyrethroids but Usherwood et al. 2005 find it is entirely ineffective against DDT.[31] Scott 2019 believes this test in Drosophila oocytes holds for oocytes in general.[31]
History
[edit]

| External audio | |
|---|---|
 | |
DDT was first synthesized in 1874 by Othmar Zeidler under the supervision of Adolf von Baeyer.[32][33] It was further described in 1929 in a dissertation by W. Bausch and in two subsequent publications in 1930.[34][35] The insecticide properties of "multiple chlorinated aliphatic or fat-aromatic alcohols with at least one trichloromethane group" were described in a patent in 1934 by Wolfgang von Leuthold.[36] DDT's insecticidal properties were not, however, discovered until 1939 by the Swiss scientist Paul Hermann Müller, who was awarded the 1948 Nobel Prize in Physiology and Medicine for his efforts.[6]
Use in the 1940s and 1950s
[edit]

DDT is the best-known of several chlorine-containing pesticides used in the 1940s and 1950s. During this time, the use of DDT was driven by protecting American soldiers from diseases in tropical areas. Both British and American scientists hoped to use it to control spread of malaria, typhus, dysentery, and typhoid fever among overseas soldiers, especially considering that the pyrethrum was harder to access since it came mainly from Japan.[37][38] Due to the potency of DDT, it was not long before America's War Production Board placed it on military supply lists in 1942 and 1943 and encouraged its production for overseas use. Enthusiasm regarding DDT became obvious through the American government's advertising campaigns of posters depicting Americans fighting the Axis powers and insects and through media publications celebrating its military uses.[37] In the South Pacific, it was sprayed aerially for malaria and dengue fever control with spectacular effects. While DDT's chemical and insecticidal properties were important factors in these victories, advances in application equipment coupled with competent organization and sufficient manpower were also crucial to the success of these programs.[39]
In 1945, DDT was made available to farmers as an agricultural insecticide[5] and played a role in the elimination of malaria in Europe and North America.[15][40][41] Despite concerns emerging in the scientific community, and lack of research, the FDA considered it safe up to 7 parts per million in food. There was a large economic incentive to push DDT into the market and sell it to farmers, governments, and individuals to control diseases and increase food production.[37]
DDT was also a way for American influence to reach abroad through DDT-spraying campaigns. In the 1944 issue of Life magazine there was a feature regarding the Italian program showing pictures of American public health officials in uniforms spraying DDT on Italian families.[37]
In 1955, the World Health Organization commenced a program to eradicate malaria in countries with low to moderate transmission rates worldwide, relying largely on DDT for mosquito control and rapid diagnosis and treatment to reduce transmission.[42] The program eliminated the disease in "North America, Europe, the former Soviet Union",[43] and in "Taiwan, much of the Caribbean, the Balkans, parts of northern Africa, the northern region of Australia, and a large swath of the South Pacific"[44] and dramatically reduced mortality in Sri Lanka and India.[45]
However, failure to sustain the program, increasing mosquito tolerance to DDT, and increasing parasite tolerance led to a resurgence. In many areas early successes partially or completely reversed, and in some cases rates of transmission increased.[13] The program succeeded in eliminating malaria only in areas with "high socio-economic status, well-organized healthcare systems, and relatively less intensive or seasonal malaria transmission".[43]
DDT was less effective in tropical regions due to the continuous life cycle of mosquitoes and poor infrastructure. It was applied in sub-Saharan Africa by various colonial states, but the 'global' WHO eradication program didn't include the region.[46] Mortality rates in that area never declined to the same dramatic extent, and now constitute the bulk of malarial deaths worldwide, especially following the disease's resurgence as a result of resistance to drug treatments and the spread of the deadly malarial variant caused by Plasmodium falciparum. Eradication was abandoned in 1969 and attention instead focused on controlling and treating the disease. Spraying programs (especially using DDT) were curtailed due to concerns over safety and environmental effects, as well as problems in administrative, managerial and financial implementation.[13] Efforts shifted from spraying to the use of bednets impregnated with insecticides and other interventions.[43][47]
United States ban
[edit]By October 1945, DDT was available for public sale in the United States, used both as an agricultural pesticide and as a household insecticide.[9] Although its use was promoted by government and the agricultural industry, US scientists such as FDA pharmacologist Herbert O. Calvery expressed concern over possible hazards associated with DDT as early as 1944.[48][24][9] In 1947, Bradbury Robinson, a physician and nutritionist practicing in St. Louis, Michigan, warned of the dangers of using the pesticide DDT in agriculture. DDT had been researched and manufactured in St. Louis by the Michigan Chemical Corporation, later purchased by Velsicol Chemical Corporation,[49] and had become an important part of the local economy.[50] Citing research performed by Michigan State University[51] in 1946, Robinson, a past president of the local Conservation Club,[52] opined that:
perhaps the greatest danger from D.D.T. is that its extensive use in farm areas is most likely to upset the natural balances, not only killing beneficial insects in great number but by bringing about the death of fish, birds, and other forms of wild life either by their feeding on insects killed by D.D.T. or directly by ingesting the poison.[53]
As its production and use increased, public response was mixed. At the same time that DDT was hailed as part of the "world of tomorrow", concerns were expressed about its potential to kill harmless and beneficial insects (particularly pollinators), birds, fish, and eventually humans. The issue of toxicity was complicated, partly because DDT's effects varied from species to species, and partly because consecutive exposures could accumulate, causing damage comparable to large doses. A number of states attempted to regulate DDT.[9][5] In the 1950s the federal government began tightening regulations governing its use.[24] These events received little attention. Women like Dorothy Colson and Mamie Ella Plyler of Claxton, Georgia, gathered evidence about DDT's effects and wrote to the Georgia Department of Public Health, the National Health Council in New York City, and other organizations.[54]
In 1957 The New York Times reported an unsuccessful struggle to restrict DDT use in Nassau County, New York, and the issue came to the attention of the popular naturalist-author Rachel Carson when a friend, Olga Huckins, wrote to her including an article she had written in the Boston Globe about the devastation of her local bird population after DDT spraying.[55][56] William Shawn, editor of The New Yorker, urged her to write a piece on the subject, which developed into her 1962 book Silent Spring. The book argued that pesticides, including DDT, were poisoning both wildlife and the environment and were endangering human health.[10] Silent Spring was a best seller, and public reaction to it launched the modern environmental movement in the United States. The year after it appeared, President John F. Kennedy ordered his Science Advisory Committee to investigate Carson's claims. The committee's report "add[ed] up to a fairly thorough-going vindication of Rachel Carson's Silent Spring thesis", in the words of the journal Science,[57] and recommended a phaseout of "persistent toxic pesticides".[58] In 1965, the U.S. military removed DDT from the military supply system due in part to the development of resistance by body lice to DDT; it was replaced by lindane.[59]
In the mid-1960s, DDT became a prime target of the burgeoning environmental movement, as concern about DDT and its effects began to rise in local communities. In 1966, a fish kill in Suffolk County, NY, was linked to a 5,000-gallon DDT dump by the county's mosquito commission, leading a group of scientists and lawyers to file a lawsuit to stop the county's further use of DDT.[60] A year later, the group, led by Victor Yannacone and Charles Wurster, founded the Environmental Defense Fund (EDF), along with scientists Art Cooley and Dennis Puleston, and brought a string of lawsuits against DDT and other persistent pesticides in Michigan and Wisconsin.[61][62]
Around the same time, evidence was mounting further about DDT causing catastrophic declines in wildlife reproduction, especially in birds of prey like peregrine falcons, bald eagles, ospreys, and brown pelicans, whose eggshells became so thin that they often cracked before hatching.[63] Toxicologists like David Peakall were measuring DDE levels in the eggs of peregrine falcons and California condors and finding that increased levels corresponded with thinner shells.[64] Compounding the effect was DDT’s persistence in the environment, as it was unable to dissolve in water, and ended up accumulating in animal fat and disrupting hormone metabolism across a wide range of species.[65]
In response to an EDF suit, the U.S. District Court of Appeals in 1971 ordered the EPA to begin the de-registration procedure for DDT. After an initial six-month review process, William Ruckelshaus, the Agency's first Administrator rejected an immediate suspension of DDT's registration, citing studies from the EPA's internal staff stating that DDT was not an imminent danger.[24] However, these findings were criticized, as they were performed mostly by economic entomologists inherited from the United States Department of Agriculture, who many environmentalists felt were biased towards agribusiness and understated concerns about human health and wildlife. The decision thus created controversy.[39]
The EPA held seven months of hearings in 1971–1972, with scientists giving evidence for and against DDT. In the summer of 1972, Ruckelshaus announced the cancellation of most uses of DDT – exempting public health uses under some conditions.[24] Again, this caused controversy. Immediately after the announcement, both the EDF and the DDT manufacturers filed suit against EPA. Many in the agricultural community were concerned that food production would be severely impacted, while proponents of pesticides warned of increased breakouts of insect-borne diseases and questioned the accuracy of giving animals high amounts of pesticides for cancer potential.[66] Industry sought to overturn the ban, while the EDF wanted a comprehensive ban. The cases were consolidated, and in 1973 the United States Court of Appeals for the District of Columbia Circuit ruled that the EPA had acted properly in banning DDT.[24] During the late 1970s, the EPA also began banning organochlorines, pesticides that were chemically similar to DDT. These included aldrin, dieldrin, chlordane, heptachlor, toxaphene, and mirex.[66]
Some uses of DDT continued under the public health exemption. For example, in June 1979, the California Department of Health Services was permitted to use DDT to suppress flea vectors of bubonic plague.[67] DDT continued to be produced in the United States for foreign markets until 1985, when over 300 tons were exported.[1]
International usage restrictions
[edit]In the 1970s and 1980s, agricultural use was banned in most developed countries, beginning with Hungary in 1968[68][69][70] – although in practice it continued to be used through at least 1970.[71] This was followed by Norway and Sweden in 1970, West Germany and the United States in 1972, but not in the United Kingdom until 1984.
In contrast to West Germany, in the German Democratic Republic DDT was used until 1988. Especially of relevance were large-scale applications in forestry in the years 1982–1984, with the aim to combat bark beetle and pine moth. As a consequence, DDT-concentrations in eastern German forest soils are still significantly higher compared to soils in the former western German states.[72]
By 1991, total bans, including for disease control, were in place in at least 26 countries; for example, Cuba in 1970, the US in the 1980s, Singapore in 1984, Chile in 1985, and the Republic of Korea in 1986.[73]
The Stockholm Convention on Persistent Organic Pollutants, which took effect in 2004, put a global ban on several persistent organic pollutants, and restricted DDT use to vector control. The convention was ratified by more than 170 countries. Recognizing that total elimination in many malaria-prone countries is currently unfeasible in the absence of affordable/effective alternatives, the convention exempts public health use within World Health Organization (WHO) guidelines from the ban.[14] Resolution 60.18 of the World Health Assembly commits WHO to the Stockholm Convention's aim of reducing and ultimately eliminating DDT.[74] Malaria Foundation International states, "The outcome of the treaty is arguably better than the status quo going into the negotiations. For the first time, there is now an insecticide which is restricted to vector control only, meaning that the selection of resistant mosquitoes will be slower than before."[75]
Despite the worldwide ban, agricultural use continued in India,[76] North Korea, and possibly elsewhere.[7] As of 2013, an estimated 3,000 to 4,000 tons of DDT were produced for disease vector control, including 2,786 tons in India.[77] DDT is applied to the inside walls of homes to kill or repel mosquitoes. This intervention, called indoor residual spraying (IRS), greatly reduces environmental damage. It also reduces the incidence of DDT resistance.[78] For comparison, treating 40 hectares (99 acres) of cotton during a typical U.S. growing season requires the same amount of chemical to treat roughly 1,700 homes.[79]
Environmental impact
[edit]
DDT is a persistent organic pollutant that is readily adsorbed to soils and sediments, which can act both as sinks and as long-term sources of exposure affecting organisms.[19] Depending on environmental conditions, its soil half-life can range from 22 days to 30 years. Routes of loss and degradation include runoff, volatilization, photolysis and aerobic and anaerobic biodegradation. Due to hydrophobic properties, in aquatic ecosystems DDT and its metabolites are absorbed by aquatic organisms and adsorbed on suspended particles, leaving little DDT dissolved in the water (however, its half-life in aquatic environments is listed by the National Pesticide Information Center as 150 years[80]). Its breakdown products and metabolites, DDE and DDD, are also persistent and have similar chemical and physical properties.[1] DDT and its breakdown products are transported from warmer areas to the Arctic by the phenomenon of global distillation, where they then accumulate in the region's food web.[81]
Medical researchers in 1974 found a measurable and significant difference in the presence of DDT in human milk between mothers who lived in New Brunswick and mothers who lived in Nova Scotia, "possibly because of the wider use of insecticide sprays in the past".[82]
Because of its lipophilic properties, DDT can bioaccumulate, especially in predatory birds.[83] DDT is toxic to a wide range of living organisms, including marine animals such as crayfish, daphnids, sea shrimp and many species of fish. DDT, DDE and DDD magnify through the food chain, with apex predators such as raptor birds concentrating more chemicals than other animals in the same environment. They are stored mainly in body fat. DDT and DDE are resistant to metabolism; in humans, their half-lives are 6 and up to 10 years, respectively. In the United States, these chemicals were detected in almost all human blood samples tested by the Centers for Disease Control in 2005, though their levels have sharply declined since most uses were banned.[84] Estimated dietary intake has declined,[84] although FDA food tests commonly detect it.[85]
Despite being banned for many years, in 2018 research showed that DDT residues are still present in European soils and Spanish rivers.[86][87]
Eggshell thinning
[edit]The chemical and its breakdown products DDE and DDD caused eggshell thinning and population declines in multiple North American and European bird of prey species.[1][88][11][89][90][91] Both laboratory experiments and field studies confirmed this effect.[92] The effect was first conclusively proven at Bellow Island in Lake Michigan during University of Michigan-funded studies on American herring gulls in the mid-1960s.[93] DDE-related eggshell thinning is considered a major reason for the decline of the bald eagle,[11] brown pelican,[94] peregrine falcon and osprey.[1] However, birds vary in their sensitivity to these chemicals, with birds of prey, waterfowl and song birds being more susceptible than chickens and related species.[1][19] Even in 2010, California condors that feed on sea lions at Big Sur that in turn feed in the Palos Verdes Shelf area of the Montrose Chemical Superfund site exhibited continued thin-shell problems,[95][96] though DDT's role in the decline of the California condor is disputed.[91][90]
The biological thinning mechanism is not entirely understood, but DDE appears to be more potent than DDT,[1] and strong evidence indicates that p,p'-DDE inhibits calcium ATPase in the membrane of the shell gland and reduces the transport of calcium carbonate from blood into the eggshell gland. This results in a dose-dependent thickness reduction.[1][97][98][89] Other evidence indicates that o,p'-DDT disrupts female reproductive tract development, later impairing eggshell quality.[99] Multiple mechanisms may be at work, or different mechanisms may operate in different species.[1]
Human health
[edit]


DDT is an endocrine disruptor.[100][101] It is considered likely to be a human carcinogen although the majority of studies suggest it is not directly genotoxic.[102][103][104] DDE acts as a weak androgen receptor antagonist, but not as an estrogen.[105] p,p'-DDT, DDT's main component, has little or no androgenic or estrogenic activity.[106] The minor component o,p'-DDT has weak estrogenic activity.
Acute toxicity
[edit]DDT is classified as "moderately toxic" by the U.S. National Toxicology Program (NTP) and "moderately hazardous" by WHO, based on the rat oral LD50 of 113 mg/kg.[107] Indirect exposure is considered relatively non-toxic for humans.[108]
Chronic toxicity
[edit]Primarily through the tendency for DDT to build up in areas of the body with high lipid content, chronic exposure can affect reproductive capabilities and the embryo or fetus.[108]
- A review article in The Lancet states: "research has shown that exposure to DDT at amounts that would be needed in malaria control might cause preterm birth and early weaning ... toxicological evidence shows endocrine-disrupting properties; human data also indicate possible disruption in semen quality, menstruation, gestational length, and duration of lactation".[47]
- Other studies document decreases in semen quality among men with high exposures (generally from indoor residual spraying).[109]
- Studies are inconsistent on whether high blood DDT or DDE levels increase time to pregnancy.[84] In mothers with high DDE blood serum levels, daughters may have up to a 32% increase in the probability of conceiving, but increased DDT levels have been associated with a 16% decrease in one study.[110]
- Indirect exposure of mothers through workers directly in contact with DDT is associated with an increase in spontaneous abortions.[108]
- Other studies found that DDT or DDE interfere with proper thyroid function in pregnancy and childhood.[84][111]
- Mothers with high levels of DDT circulating in their blood during pregnancy were found to be more likely to give birth to children who would go on to develop autism.[112][113]
Carcinogenicity
[edit]In 2015, the International Agency for Research on Cancer classified DDT as Group 2A "probably carcinogenic to humans".[114] Previous assessments by the U.S. National Toxicology Program classified it as "reasonably anticipated to be a carcinogen" and by the EPA classified DDT, DDE and DDD as class B2 "probable" carcinogens; these evaluations were based mainly on animal studies.[1][47]
A 2005 Lancet review stated that occupational DDT exposure was associated with increased pancreatic cancer risk in 2 case control studies, but another study showed no DDE dose-effect association. Results regarding a possible association with liver cancer and biliary tract cancer are conflicting: workers who did not have direct occupational DDT contact showed increased risk. White men had an increased risk, but not white women or black men. Results about an association with multiple myeloma, prostate and testicular cancer, endometrial cancer and colorectal cancer have been inconclusive or generally do not support an association.[47] A 2017 review of liver cancer studies concluded that "organochlorine pesticides, including DDT, may increase hepatocellular carcinoma risk".[115]
A 2009 review, whose co-authors included persons engaged in DDT-related litigation, reached broadly similar conclusions, with an equivocal association with testicular cancer. Case–control studies did not support an association with leukemia or lymphoma.[84]
Breast cancer
[edit]The question of whether DDT or DDE are risk factors in breast cancer has not been conclusively answered. Several meta analyses of observational studies have concluded that there is no overall relationship between DDT exposure and breast cancer risk.[116][117] The United States Institute of Medicine reviewed data on the association of breast cancer with DDT exposure in 2012 and concluded that a causative relationship could neither be proven nor disproven.[118]
A 2007 case-control study[106] using archived blood samples found that breast cancer risk was increased 5-fold among women who were born prior to 1931 and who had high serum DDT levels in 1963. Reasoning that DDT use became widespread in 1945 and peaked around 1950, they concluded that the ages of 14–20 were a critical period in which DDT exposure leads to increased risk. This study, which suggests a connection between DDT exposure and breast cancer that would not be picked up by most studies, has received variable commentary in third-party reviews. One review suggested that "previous studies that measured exposure in older women may have missed the critical period".[84][119] The National Toxicology Program notes that while the majority of studies have not found a relationship between DDT exposure and breast cancer that positive associations have been seen in a "few studies among women with higher levels of exposure and among certain subgroups of women".[103]
A 2015 case control study identified a link (odds ratio 3.4) between in-utero exposure (as estimated from archived maternal blood samples) and breast cancer diagnosis in daughters. The findings "support classification of DDT as an endocrine disruptor, a predictor of breast cancer, and a marker of high risk".[120]
Malaria control
[edit]Malaria remains the primary public health challenge in many countries. In 2015, there were 214 million cases of malaria worldwide resulting in an estimated 438,000 deaths, 90% of which occurred in Africa.[121] DDT is one of many tools to fight the disease. Its use in this context has been called everything from a "miracle weapon [that is] like Kryptonite to the mosquitoes",[122] to "toxic colonialism".[123]
Before DDT, eliminating mosquito breeding grounds by drainage or poisoning with Paris green or pyrethrum was sometimes successful. In parts of the world with rising living standards, the elimination of malaria was often a collateral benefit of the introduction of window screens and improved sanitation.[44] A variety of usually simultaneous interventions represents best practice. These include antimalarial drugs to prevent or treat infection; improvements in public health infrastructure to diagnose, sequester and treat infected individuals; bednets and other methods intended to keep mosquitoes from biting humans; and vector control strategies[124] such as larviciding with insecticides, ecological controls such as draining mosquito breeding grounds or introducing fish to eat larvae and indoor residual spraying (IRS) with insecticides, possibly including DDT. IRS involves the treatment of interior walls and ceilings with insecticides. It is particularly effective against mosquitoes, since many species rest on an indoor wall before or after feeding. DDT is one of 12 WHO–approved IRS insecticides.[43]
The WHO's anti-malaria campaign of the 1950s and 1960s relied heavily on DDT and the results were promising, though temporary in developing countries. Experts tie malarial resurgence to multiple factors, including poor leadership, management and funding of malaria control programs; poverty; civil unrest; and increased irrigation. The evolution of resistance to first-generation drugs (e.g. chloroquine) and to insecticides exacerbated the situation.[7][8] Resistance was largely fueled by unrestricted agricultural use. Resistance and the harm both to humans and the environment led many governments to curtail DDT use in vector control and agriculture.[13] In 2006 WHO reversed a longstanding policy against DDT by recommending that it be used as an indoor pesticide in regions where malaria is a major problem.[125]
Once the mainstay of anti-malaria campaigns, as of 2019 only five countries used DDT for Indoor Residual Spraying [126]
Initial effectiveness
[edit]When it was introduced in World War II, DDT was effective in reducing malaria morbidity and mortality.[39] WHO's anti-malaria campaign, which consisted mostly of spraying DDT and rapid treatment and diagnosis to break the transmission cycle, was initially successful as well. For example, in Sri Lanka, the program reduced cases from about one million per year before spraying to just 18 in 1963[127][128] and 29 in 1964. Thereafter the program was halted to save money and malaria rebounded to 600,000 cases in 1968 and the first quarter of 1969. The country resumed DDT vector control but the mosquitoes had evolved resistance in the interim, presumably because of continued agricultural use. The program switched to malathion, but despite initial successes, malaria continued its resurgence into the 1980s.[45][129]
DDT remains on WHO's list of insecticides recommended for IRS. After the appointment of Arata Kochi as head of its anti-malaria division, WHO's policy shifted from recommending IRS only in areas of seasonal or episodic transmission of malaria, to advocating it in areas of continuous, intense transmission.[130] WHO reaffirmed its commitment to phasing out DDT, aiming "to achieve a 30% cut in the application of DDT world-wide by 2014 and its total phase-out by the early 2020s if not sooner" while simultaneously combating malaria. WHO plans to implement alternatives to DDT to achieve this goal.[131]
South Africa continues to use DDT under WHO guidelines. In 1996, the country switched to alternative insecticides and malaria incidence increased dramatically. Returning to DDT and introducing new drugs brought malaria back under control.[132] Malaria cases increased in South America after countries in that continent stopped using DDT. Research data showed a strong negative relationship between DDT residual house sprayings and malaria. In a research from 1993 to 1995, Ecuador increased its use of DDT and achieved a 61% reduction in malaria rates, while each of the other countries that gradually decreased its DDT use had large increases.[79][133][134]
Mosquito resistance
[edit]In some areas, resistance reduced DDT's effectiveness. WHO guidelines require that absence of resistance must be confirmed before using the chemical.[17] Resistance is largely due to agricultural use, in much greater quantities than required for disease prevention.
Resistance was noted early in spray campaigns. Paul Russell, former head of the Allied Anti-Malaria campaign, observed in 1956 that "resistance has appeared after six or seven years".[44] Resistance has been detected in Sri Lanka, Pakistan, Turkey and Central America and it has largely been replaced by organophosphate or carbamate insecticides, e.g. malathion or bendiocarb.[135]
In many parts of India, DDT is ineffective.[136] Agricultural uses were banned in 1989 and its anti-malarial use has been declining. Urban use ended.[137] One study concluded that "DDT is still a viable insecticide in indoor residual spraying owing to its effectivity in well supervised spray operation and high excito-repellency factor."[138]
Studies of malaria-vector mosquitoes in KwaZulu-Natal Province, South Africa found susceptibility to 4% DDT (WHO's susceptibility standard), in 63% of the samples, compared to the average of 87% in the same species caught in the open. The authors concluded that "Finding DDT resistance in the vector An. arabiensis, close to the area where we previously reported pyrethroid-resistance in the vector An. funestus Giles, indicates an urgent need to develop a strategy of insecticide resistance management for the malaria control programmes of southern Africa."[139]
DDT can still be effective against resistant mosquitoes[140] and the avoidance of DDT-sprayed walls by mosquitoes is an additional benefit of the chemical.[138] For example, a 2007 study reported that resistant mosquitoes avoided treated huts. The researchers argued that DDT was the best pesticide for use in IRS (even though it did not afford the most protection from mosquitoes out of the three test chemicals) because the other pesticides worked primarily by killing or irritating mosquitoes – encouraging the development of resistance.[140] Others argue that the avoidance behavior slows eradication.[141] Unlike other insecticides such as pyrethroids, DDT requires long exposure to accumulate a lethal dose; however its irritant property shortens contact periods. "For these reasons, when comparisons have been made, better malaria control has generally been achieved with pyrethroids than with DDT."[135] In India outdoor sleeping and night duties are common, implying that "the excito-repellent effect of DDT, often reported useful in other countries, actually promotes outdoor transmission".[142]
Residents' concerns
[edit]IRS is effective if at least 80% of homes and barns in a residential area are sprayed.[17] Lower coverage rates can jeopardize program effectiveness. Many residents resist DDT spraying, objecting to the lingering smell, stains on walls, and the potential exacerbation of problems with other insect pests.[135][141][143] Pyrethroid insecticides (e.g. deltamethrin and lambda-cyhalothrin) can overcome some of these issues, increasing participation.[135]
Human exposure
[edit]A 1994 study found that South Africans living in sprayed homes have levels that are several orders of magnitude greater than others.[84] Breast milk from South African mothers contains high levels of DDT and DDE.[84] It is unclear to what extent these levels arise from home spraying vs food residues. Evidence indicates that these levels are associated with infant neurological abnormalities.[135]
Most studies of DDT's human health effects have been conducted in developed countries where DDT is not used and exposure is relatively low.[47][84][144]
Illegal diversion to agriculture is also a concern as it is difficult to prevent and its subsequent use on crops is uncontrolled. For example, DDT use is widespread in Indian agriculture,[145] particularly mango production[146] and is reportedly used by librarians to protect books.[147] Other examples include Ethiopia, where DDT intended for malaria control is reportedly used in coffee production,[148] and Ghana where it is used for fishing.[149][150] The residues in crops at levels unacceptable for export have been an important factor in bans in several tropical countries.[135] Adding to this problem is a lack of skilled personnel and management.[141]
Criticism of restrictions on DDT use
[edit]Restrictions on DDT usage have been criticized by some organizations opposed to the environmental movement, including Roger Bate of the pro-DDT advocacy group Africa Fighting Malaria and the libertarian think tank Competitive Enterprise Institute; these sources oppose restrictions on DDT and attribute large numbers of deaths to such restrictions, sometimes in the millions.[151][152][153] These arguments were rejected as "outrageous" by former WHO scientist Socrates Litsios.[122] May Berenbaum, University of Illinois entomologist, says, "to blame environmentalists who oppose DDT for more deaths than Hitler is worse than irresponsible".[122] More recently, Michael Palmer, a professor of chemistry at the University of Waterloo, has pointed out that DDT is still used to prevent malaria, that its declining use is primarily due to increases in manufacturing costs, and that in Africa, efforts to control malaria have been regional or local, not comprehensive.[154]
The question that ... malaria control experts must ask is not "Which is worse, malaria or DDT?" but rather "What are the best tools to deploy for malaria control in a given situation, taking into account the on-the-ground challenges and needs, efficacy, cost, and collateral effects – both positive and negative – to human health and the environment, as well as the uncertainties associated with all these considerations?"
Criticisms of a DDT "ban" often specifically reference the 1972 United States ban (with the erroneous implication that this constituted a worldwide ban and prohibited use of DDT in vector control). Reference is often made to Silent Spring, even though Carson never pushed for a DDT ban. John Quiggin and Tim Lambert wrote, "the most striking feature of the claim against Carson is the ease with which it can be refuted".[156]
Investigative journalist Adam Sarvana and others characterize these notions as "myths" promoted principally by Roger Bate of the pro-DDT advocacy group Africa Fighting Malaria (AFM).[157][158]
Alternatives
[edit]Insecticides
[edit]Organophosphate and carbamate insecticides, e.g. malathion and bendiocarb, respectively, are more expensive than DDT per kilogram and are applied at roughly the same dosage. Pyrethroids such as deltamethrin are also more expensive than DDT, but are applied more sparingly (0.02–0.3 g/m2 vs 1–2 g/m2), so the net cost per house per treatment is about the same.[43] DDT has one of the longest residual efficacy periods of any IRS insecticide, lasting 6 to 12 months. Pyrethroids will remain active for only 4 to 6 months, and organophosphates and carbamates remain active for 2 to 6 months. In many malaria-endemic countries, malaria transmission occurs year-round, meaning that the high expense of conducting a spray campaign (including hiring spray operators, procuring insecticides, and conducting pre-spray outreach campaigns to encourage people to be home and to accept the intervention) will need to occur multiple times per year for these shorter-lasting insecticides.[159]
In 2019, the related compound difluorodiphenyltrichloroethane (DFDT) was described as a potentially more effective and therefore potentially safer alternative to DDT.[160][161]
Non-chemical vector control
[edit]Before DDT, malaria was successfully eliminated or curtailed in several tropical areas by removing or poisoning mosquito breeding grounds and larva habitats, for example by eliminating standing water. These methods have seen little application in Africa for more than half a century.[162] According to CDC, such methods are not practical in Africa because "Anopheles gambiae, one of the primary vectors of malaria in Africa, breeds in numerous small pools of water that form due to rainfall ... It is difficult, if not impossible, to predict when and where the breeding sites will form, and to find and treat them before the adults emerge."[163]
The relative effectiveness of IRS versus other malaria control techniques (e.g. bednets or prompt access to anti-malarial drugs) varies and is dependent on local conditions.[43]
A WHO study released in January 2008 found that mass distribution of insecticide-treated mosquito nets and artemisinin–based drugs cut malaria deaths in half in malaria-burdened Rwanda and Ethiopia. IRS with DDT did not play an important role in mortality reduction in these countries.[164][165]
Vietnam has enjoyed declining malaria cases and a 97% mortality reduction after switching in 1991 from a poorly funded DDT-based campaign to a program based on prompt treatment, bednets and pyrethroid group insecticides.[166]
In Mexico, effective and affordable chemical and non-chemical strategies were so successful that the Mexican DDT manufacturing plant ceased production due to lack of demand.[167]
A review of fourteen studies in sub-Saharan Africa, covering insecticide-treated nets, residual spraying, chemoprophylaxis for children, chemoprophylaxis or intermittent treatment for pregnant women, a hypothetical vaccine and changing front–line drug treatment, found decision making limited by the lack of information on the costs and effects of many interventions, the small number of cost-effectiveness analyses, the lack of evidence on the costs and effects of packages of measures and the problems in generalizing or comparing studies that relate to specific settings and use different methodologies and outcome measures. The two cost-effectiveness estimates of DDT residual spraying examined were not found to provide an accurate estimate of the cost-effectiveness of DDT spraying; the resulting estimates may not be good predictors of cost-effectiveness in current programs.[168]
However, a study in Thailand found the cost per malaria case prevented of DDT spraying (US$1.87) to be 21% greater than the cost per case prevented of lambda-cyhalothrin–treated nets (US$1.54),[169] casting some doubt on the assumption that DDT was the most cost-effective measure. The director of Mexico's malaria control program found similar results, declaring that it was 25% cheaper for Mexico to spray a house with synthetic pyrethroids than with DDT.[167] However, another study in South Africa found generally lower costs for DDT spraying than for impregnated nets.[170]
A more comprehensive approach to measuring the cost-effectiveness or efficacy of malarial control would not only measure the cost in dollars, as well as the number of people saved, but would also consider ecological damage and negative human health impacts. One preliminary study found that it is likely that the detriment to human health approaches or exceeds the beneficial reductions in malarial cases, except perhaps in epidemics. It is similar to the earlier study regarding estimated theoretical infant mortality caused by DDT and subject to the criticism also mentioned earlier.[171]
A study in the Solomon Islands found that "although impregnated bed nets cannot entirely replace DDT spraying without substantial increase in incidence, their use permits reduced DDT spraying".[172]
A comparison of four successful programs against malaria in Brazil, India, Eritrea and Vietnam does not endorse any single strategy but instead states, "Common success factors included conducive country conditions, a targeted technical approach using a package of effective tools, data-driven decision-making, active leadership at all levels of government, involvement of communities, decentralized implementation and control of finances, skilled technical and managerial capacity at national and sub-national levels, hands-on technical and programmatic support from partner agencies, and sufficient and flexible financing."[173]
DDT resistant mosquitoes may be susceptible to pyrethroids in some countries. However, pyrethroid resistance in Anopheles mosquitoes is on the rise with resistant mosquitoes found in multiple countries.[174]
See also
[edit]- DDT in New Zealand
- Operation Cat Drop
- Environmental hazard
- Index of pesticide articles
- Mosquito control
References
[edit]- ^ a b c d e f g h i j k l m Toxicological Profile: for DDT, DDE, and DDE Archived November 25, 2021, at the Wayback Machine. Agency for Toxic Substances and Disease Registry, September 2002.
- ^ "DDT". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0174". National Institute for Occupational Safety and Health (NIOSH).
- ^ "NIOSH Pocket Guide to Chemical Hazards".
- ^ a b c d e f DDT and its derivatives, Environmental Health Criteria monograph No. 009, Geneva: World Health Organization, 1979, ISBN 92-4-154069-9
- ^ a b "The Nobel Prize in Physiology of Medicine 1948". Nobel Prize Outreach AB. Archived from the original on May 23, 2020. Retrieved July 26, 2007.
- ^ a b c d van den Berg H (October 23, 2008). "Global status of DDT and its alternatives for use in vector control to prevent disease" (PDF). Stockholm Convention on Persistent Organic Pollutants/United Nations Environment Programme. Archived from the original (PDF) on December 17, 2010. Retrieved November 22, 2008.
- ^ a b Feachem RG, Sabot OJ (May 2007). "Global malaria control in the 21st century: a historic but fleeting opportunity". JAMA. 297 (20): 2281–2284. doi:10.1001/jama.297.20.2281. PMID 17519417.
- ^ a b c d Conis, Elena (2017). "Beyond Silent Spring: An Alternate History of DDT". Distillations. 2 (4): 16–23. Archived from the original on November 22, 2019. Retrieved March 20, 2018.
- ^ a b Lear, Linda (2009). Rachel Carson: Witness for Nature. Mariner Books. ISBN 978-0-547-23823-4. Archived from the original on October 19, 2021. Retrieved August 29, 2022.
- ^ a b c Stokstad E (June 2007). "Species conservation. Can the bald eagle still soar after it is delisted?". Science. 316 (5832): 1689–1690. doi:10.1126/science.316.5832.1689. PMID 17588911. S2CID 5051469.
- ^ United States Fish and Wildlife Service, Fact Sheet: Natural History, Ecology, and History of Recovery [1] Archived May 21, 2020, at the Wayback Machine
- ^ a b c d Chapin G, Wasserstrom R (1981). "Agricultural production and malaria resurgence in Central America and India". Nature. 293 (5829): 181–185. Bibcode:1981Natur.293..181C. doi:10.1038/293181a0. PMID 7278974. S2CID 4346743.
- ^ a b "Stockholm Convention on Persistent Organic Pollutants" (PDF). Archived (PDF) from the original on June 5, 2015. Retrieved August 24, 2014.
- ^ a b Larson K (December 1, 2007). "Bad Blood". On Earth (Winter 2008). Archived from the original on April 13, 2020. Retrieved June 5, 2008.
- ^ Moyers B (September 21, 2007). "Rachel Carson and DDT". Bill Moyers Journal. PBS. Archived from the original on April 25, 2011. Retrieved March 5, 2011.
- ^ a b c d " Indoor Residual Spraying: Use of Indoor Residual Spraying for Scaling Up Global Malaria Control and Elimination Archived October 2, 2008, at the Wayback Machine". World Health Organization, 2006.
- ^ Lammel, G.; et al. (December 2011). "Sources of organochlorine pesticides in air in an urban Mediterranean environment: volatilisation from soil". J. Environ. Monit. 13 (12): 3358–3364. doi:10.1039/c1em10479a. PMID 22020740. S2CID 22071869.
- ^ a b c DDT and Its Derivatives: Environmental Aspects, Environmental Health Criteria monograph No. 83, Geneva: World Health Organization, 1989, ISBN 9241542837
- ^ Geisz HN, Dickhut RM, Cochran MA, Fraser WR, Ducklow HW (June 2008). "Melting glaciers: a probable source of DDT to the Antarctic marine ecosystem". Environmental Science & Technology. 42 (11): 3958–3962. Bibcode:2008EnST...42.3958G. doi:10.1021/es702919n. PMID 18589951. Archived from the original on August 29, 2022. Retrieved August 26, 2020.
- ^ David D (July 4, 2008). "McIntosh residents file suit against Ciba". Archived from the original on August 8, 2009. Retrieved July 7, 2008.
- ^ Environmental Cleanup Site Information Database for Arkema (former Pennwalt) facility Archived July 23, 2011, at the Wayback Machine, Oregon DEQ, April 2009.
- ^ Horvath R (January 27, 2008). "Tests shed light on how pCBSA got into St. Louis water". Morning Sun. Michigan, United States: Journal Register Company. Archived from the original on July 5, 2008. Retrieved May 16, 2008.
- ^ a b c d e f "DDT Regulatory History: A Brief Survey (to 1975)" Archived December 20, 2016, at the Wayback Machine, U.S. EPA, July 1975.
- ^ "Report of the Third Expert Group Meeting on DDT". UNEP/POPS/DDT-EG.3/3, Stockholm Convention on Persistent Organic Pollutants. November 12, 2010. Archived from the original on December 21, 2010. Retrieved January 26, 2011.
- ^ "Alternatives to DDT". UNEP - UN Environment Programme. September 13, 2017. Retrieved June 10, 2024.
- ^ a b Dong, Ke (January 6, 2007). "Insect sodium channels and insecticide resistance". Invertebrate Neuroscience. 7 (1): 17–30. doi:10.1007/s10158-006-0036-9. ISSN 1354-2516. PMC 3052376. PMID 17206406.
- ^ Denholm I, Devine GJ, Williamson MS (September 2002). "Insecticide resistance on the move". Science. 297 (5590): 2222–2223. doi:10.1126/science.1077266. PMID 12351778. S2CID 83741532.
- ^ Pedra JH, McIntyre LM, Scharf ME, Pittendrigh BR (May 2004). "Genome-wide transcription profile of field- and laboratory-selected dichlorodiphenyltrichloroethane (DDT)-resistant Drosophila". Proceedings of the National Academy of Sciences of the United States of America. 101 (18): 7034–7039. Bibcode:2004PNAS..101.7034P. doi:10.1073/pnas.0400580101. PMC 406461. PMID 15118106.
- ^ Bijlsma, R.; Loeschcke, Volker (November 7, 2011). "Genetic erosion impedes adaptive responses to stressful environments". Evolutionary Applications. 5 (2). Blackwell: 117–129. doi:10.1111/j.1752-4571.2011.00214.x. ISSN 1752-4571. PMC 3353342. PMID 25568035. S2CID 18877551.
- ^ a b Scott, Jeffrey G. (January 7, 2019). "Life and Death at the Voltage-Sensitive Sodium Channel: Evolution in Response to Insecticide Use". Annual Review of Entomology. 64 (1). Annual Reviews: 243–257. doi:10.1146/annurev-ento-011118-112420. ISSN 0066-4170. PMID 30629893. S2CID 58667542.
- ^ Othmar Zeidler (1874). "Verbindungen von Chloral mit Brom- und Chlorbenzol" [Compounds of chloral with bromo- and chlorobenzene]. Berichte der Deutschen Chemischen Gesellschaft. 7 (2): 1180–1181. doi:10.1002/cber.18740070278. Archived from the original on April 20, 2016. On p. 1181, Zeidler called DDT dimonochlorphenyltrichloräthan.
- ^ Augustin F (1993). Zur Geschichte des Insektizids Dichlordiphenyltrichloräthan (DDT) unter besonderer Berücksichtigung der Leistung des Chemikers Paul Müller (1899–1965). Leipzig: Medizinische Fakultät der Universität Leipzig. pp. 1–77. Archived from the original on July 31, 2020. Retrieved August 29, 2022.
- ^ Brand K, Bausch W (1930). "Über Verbindungen der Tetraaryl-butanreihe. 10. Mitteilung. Über die Reduktion organischer Halogenverbindungen und Über Verbindungen der Tetraaryl-butanreihe". Journal für Praktische Chemie. 127: 219–239. doi:10.1002/prac.19301270114.
- ^ Brand K, Horn O, Bausch W (1930). "Die elektrochemische Darstellung von 1,1,4,4-p,p′,p",p‴-Tetraphenetyl-butin-2 und von 1,1,4,4-p,p′,p",p‴-Tetra(chlorphenyl)-butin-2. 11. Mitteilung. Über die Reduktion organischer Halogenverbindungen und Verbindungen der Tetraarylbutanreihe". Journal für Praktische Chemie. 127: 240–247. doi:10.1002/prac.19301270115.
- ^ Wolfgang von Leuthold, Schädlingsbekämpfung. DRP Nr 673246, April 27, 1934
- ^ a b c d Sonnenberg, J. (May 2, 2015). "Shoot to Kill: Control and Controversy in the History of DDT Science". Stanford Journal of Public Health. Archived from the original on August 29, 2022. Retrieved April 9, 2022.
- ^ "The Deadly Dust: The Unhappy History Of DDT". AMERICAN HERITAGE. Archived from the original on April 9, 2022. Retrieved April 9, 2022.
- ^ a b c Dunlap, Thomas (2014). DDT: Scientists, Citizens, and Public Policy. Princeton University Press. ISBN 978-1-4008-5385-4. Archived from the original on October 19, 2021. Retrieved August 29, 2022.
- ^ de Zulueta J (June 1998). "The end of malaria in Europe: an eradication of the disease by control measures". Parassitologia. 40 (1–2): 245–246. PMID 9653750.
- ^ "About Malaria – History – Elimination of Malaria in the United States (1947–1951)". CDC.gov. January 28, 2019. Archived from the original on May 4, 2012. Retrieved September 9, 2017.
- ^ Mendis K, Rietveld A, Warsame M, Bosman A, Greenwood B, Wernsdorfer WH (July 2009). "From malaria control to eradication: The WHO perspective". Tropical Medicine & International Health. 14 (7): 802–809. doi:10.1111/j.1365-3156.2009.02287.x. PMID 19497083. S2CID 31335358.
- ^ a b c d e f Sadasivaiah S, Tozan Y, Breman JG (December 2007). "Dichlorodiphenyltrichloroethane (DDT) for indoor residual spraying in Africa: how can it be used for malaria control?". The American Journal of Tropical Medicine and Hygiene. 77 (6 Suppl): 249–263. doi:10.4269/ajtmh.2007.77.249. PMID 18165500.
- ^ a b c Gladwell M (July 2, 2001). "The Mosquito Killer". The New Yorker. Archived from the original on April 16, 2016. Retrieved August 20, 2014.
- ^ a b Harrison GA (1978). Mosquitoes, Malaria, and Man: A History of the Hostilities Since 1880. Dutton. ISBN 978-0-525-16025-0. Archived from the original on October 19, 2021. Retrieved August 29, 2022.
- ^ Clarke, Sabine (2012). "Rethinking the Post-War Hegemony of DDT: Insecticides Research and the British Colonial Empire". In Berridge, Virginia; Gorsky, Martin (eds.). Environment, Health and History. London: Palgrave Macmillan. pp. 133–153. ISBN 978-1-349-31322-8.
- ^ a b c d e Rogan WJ, Chen A (2005). "Health risks and benefits of bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT)". Lancet. 366 (9487): 763–773. doi:10.1016/S0140-6736(05)67182-6. PMID 16125595. S2CID 3762435. Archived from the original on October 17, 2019. Retrieved June 13, 2019.
- ^ Davis, Frederick Rowe (2014). Banned : a history of pesticides and the science of toxicology. [S.l.]: Yale University Press. p. 26. ISBN 978-0300205176. Archived from the original on July 31, 2020. Retrieved July 25, 2017.
- ^ "Leading Chemical Company – Manufacture, Distribution & Sales". Velsicol Chemical, LLC. Archived from the original on October 16, 2017. Retrieved October 23, 2017.
- ^ "History by Decades". www.stlouismi.com. Archived from the original on November 18, 2006. Retrieved October 23, 2017.
- ^ American Potato Journal, June 1947, volume 24, issue 6, pp. 183–187. Results of spraying and dusting potatoes in Michigan in 1946.
- ^ "Conservation Club, St. Louis, Has Program", Lansing State Journal (Lansing, Michigan), p. 14, March 2, 1931.
- ^ Robinson B (1947). A Nutritionist Ponders the D.D.T. Problem. Private Publication (Report). St. Louis, Michigan.
- ^ Conis E (October 28, 2016). "DDT Disbelievers: Health and the New Economic Poisons in Georgia after World War II". Southern Spaces. Archived from the original on August 6, 2017. Retrieved July 25, 2017.
- ^ Knox, Robert (2012). "Duxbury celebrates Rachel Carson's 'Silent Spring'". BostonGlobe.com. Retrieved December 16, 2023.
- ^ Johnson, Jenn (February 22, 2018). "Her Heart's Home | Timeless New England". New England. Retrieved December 16, 2023.
- ^ Greenberg DS (May 1963). "Pesticides: White House Advisory Body Issues Report Recommending Steps to Reduce Hazard to Public". Science. 140 (3569): 878–879. Bibcode:1963Sci...140..878G. doi:10.1126/science.140.3569.878. PMID 17810673.
- ^ Michaels D (2008). Doubt is Their Product: How Industry's Assault on Science Threatens Your Health. New York: Oxford University Press. ISBN 978-0-19-530067-3.
- ^ "Technical Guide No. 6 – Delousing Procedures for the Control of Louse-borne Disease During Contingency Operations". United States Department of Defense Armed Forces Pest Management Board Information Services Division. November 2011.
- ^ Carter, Luther J. (December 22, 1967). "Environmental Pollution: Scientists Go to Court". Science. 158 (3808). American Association for the Advancement of Science: 1552–1556. Bibcode:1967Sci...158.1552C. doi:10.1126/science.158.3808.1552. PMID 6060359. Retrieved October 26, 2024.
- ^ Primack, J. R.; Von Hippel, Frank (1974). "The Battle Over Persistent Pesticides: From Rachel Carson to the Environmental Defense Fund" (PDF). Advice and Dissent: Scientists in the Political Arena. Basic Books. pp. 128–142. ISBN 978-0-465-00090-6.
- ^ "Sue the Bastards". Time. October 18, 1971. Archived from the original on January 19, 2012.
- ^ Henkin, Harmon; Merta, Martin; Staples, James M. (1971). The Environment, the Establishment, and the Law. Houghton Mifflin. ISBN 978-0-395-11070-6.
- ^ Peakall DB, Kiff lF (April 1979). "Eggshell thinning and dde residue levels among peregrine falcons falco peregrinus: a global perspective". Ibis. 121 (2). Wiley Online Library: 200–204. doi:10.1111/j.1474-919X.1979.tb04962.x.
- ^ Dunlap, Thomas R. (1978). "DDT on Trial: The Wisconsin Hearing, 1968-1969". Wisconsin Magazine of History. 62 (1): 3–24. ISSN 1943-7366. Retrieved December 11, 2024.
- ^ a b Susan Wayland and Penelope Fenner-Crisp. "Reducing Pesticide Risks: A Half Century of Progress" Archived April 12, 2019, at the Wayback Machine. EPA Alumni Association. March 2016.
- ^ "The Rise, Fall, Rise, and Imminent Fall of DDT". AEI. Archived from the original on January 2, 2011.
- ^ Cheremisinoff, Nicholas P.; Rosenfeld, Paul E., eds. (2011). "6 DDT and Related Compounds". Handbook of Pollution Prevention and Cleaner Production: Best Practices in the Agrochemical Industry. William Andrew. pp. 247–259. ISBN 978-1-4377-7825-0.
- ^ Nagy, B.; Vajna, L. (1972). "The Increasing Possibilities of the Application of Integrated Control in Plant Protection in Hungary". EPPO Bulletin. 2 (6). European and Mediterranean Plant Protection Organization (Wiley): 95–96. doi:10.1111/j.1365-2338.1972.tb02138.x. ISSN 0250-8052. S2CID 84111430.
- ^ "Selected passages from the history of the Hungarian plant protection administration on the 50th anniversary of establishing the county plant protection stations". Archived from the original on January 10, 2009.
- ^ Environmental Health Criteria 9 - DDT and its Derivatives. Geneva. 1979. p. 194. hdl:10665/39562. ISBN 92-4-154069-9. OCLC 67616765.
{{cite book}}: CS1 maint: location missing publisher (link) OCLC 1039198025. OCLC 504327918. ISBN 978-92-4-154069-8. OCLC 1158652149. OCLC 882544146. OCLC 5364752. - ^ Aichner, Bernhard; Bussian, Bernd; Lehnik-Habrink, Petra; Hein, Sebastian (2013). "Levels and Spatial Distribution of Persistent Organic Pollutants in the Environment: A Case Study of German Forest Soils". Environmental Science & Technology. 47 (22): 12703–12714. Bibcode:2013EnST...4712703A. doi:10.1021/es4019833. PMID 24050388.
- ^ "DDT, Decision Guidance Document, Joint FAO/UNEP Programme for the operation of Prior Informed Consent, UNEP/FAO, Rome, Italy, 1991" (PDF). Archived (PDF) from the original on April 13, 2020. Retrieved August 24, 2014.
- ^ "WHO | Strengthening malaria control while reducing reliance on DDT". WHO. Archived from the original on April 4, 2009.
- ^ "MFI second page". Malaria Foundation International. Archived from the original on October 26, 2010. Retrieved March 15, 2006.
- ^ "Concern over excessive DDT use in Jiribam fields". The Imphal Free Press. May 5, 2008. Archived from the original on December 6, 2008. Retrieved May 5, 2008.
- ^ "Report of the Sixth Expert Group Meeting on DDT". UNEP/POPS/DDT-EG.6, Stockholm Convention on Persistent Organic Pollutants. November 8, 2016. Archived from the original on March 5, 2018. Retrieved March 4, 2018.
- ^ "Is DDT still effective and needed in malaria control?". Malaria Foundation International. Archived from the original on July 21, 2011. Retrieved March 15, 2006.
- ^ a b Roberts DR, Laughlin LL, Hsheih P, Legters LJ (July–September 1997). "DDT, global strategies, and a malaria control crisis in South America". Emerging Infectious Diseases. 3 (3): 295–302. doi:10.3201/eid0303.970305. PMC 2627649. PMID 9284373.
- ^ "DDT (Technical Fact Sheet)" (PDF). Archived (PDF) from the original on February 19, 2018. Retrieved August 1, 2018.
- ^ "The Grasshopper Effect and Tracking Hazardous Air Pollutants". The Science and the Environment Bulletin (May/June 1998). Environment Canada. Archived from the original on September 28, 2004.
- ^ Musial, C. J.; Hutzinger, O.; Zitko, V.; Crocker, J. (1974). "Presence of PCB, DDE and DDT in human milk in the provinces of New Brunswick and Nova Scotia, Canada". Bulletin of Environmental Contamination and Toxicology. 12 (3): 258–267. Bibcode:1974BuECT..12..258M. doi:10.1007/BF01709117. ISSN 1432-0800. PMID 4215516.
- ^ Connell DW, Lam P, Richardson B, Wu R (1999). Introduction to Ecotoxicology. Blackwell Science. p. 68. ISBN 978-0-632-03852-7. Archived from the original on March 19, 2015. Retrieved August 29, 2022.
- ^ a b c d e f g h i Eskenazi B, Chevrier J, Rosas LG, Anderson HA, Bornman MS, Bouwman H, Chen A, Cohn BA, de Jager C, Henshel DS, Leipzig F, Leipzig JS, Lorenz EC, Snedeker SM, Stapleton D (September 2009). "The Pine River statement: human health consequences of DDT use". Environmental Health Perspectives. 117 (9): 1359–1367. Bibcode:2009EnvHP.117.1359E. doi:10.1289/ehp.11748. PMC 2737010. PMID 19750098.
- ^ USDA, Pesticide Data Program Annual Summary Calendar YearPesticide Data Program Annual Summary Calendar Year 2005, November 2006.
- ^ Silva V, Mol HG, Zomer P, Tienstra M, Ritsema CJ, Geissen V (February 2019). "Pesticide residues in European agricultural soils – A hidden reality unfolded". The Science of the Total Environment. 653: 1532–1545. Bibcode:2019ScTEn.653.1532S. doi:10.1016/j.scitotenv.2018.10.441. PMID 30759587.
- ^ Roman D, Lysimachou A, Balaguer R, Dimastrogiovanni G, García K, González E. "Ríos hormonados: Contamination of Spanish Rivers with Pesticides". Pesticide Action Network Europe. Archived from the original on February 27, 2019. Retrieved February 26, 2019.
- ^ Vos JG, Dybing E, Greim HA, Ladefoged O, Lambré C, Tarazona JV, Brandt I, Vethaak AD (January 2000). "Health effects of endocrine-disrupting chemicals on wildlife, with special reference to the European situation". Critical Reviews in Toxicology. 30 (1): 71–133. doi:10.1080/10408440091159176. PMID 10680769. S2CID 11908661.
- ^ a b Lundholm CD (October 1997). "DDE-induced eggshell thinning in birds: effects of p,p'-DDE on the calcium and prostaglandin metabolism of the eggshell gland". Comparative Biochemistry and Physiology C. 118 (2): 113–128. doi:10.1016/S0742-8413(97)00105-9. PMID 9490182.
- ^ a b Tubbs CW (2016). "California condors and DDT: Examining the effects of endocrine disrupting chemicals in a critically endangered species". Endocrine Disruptors. 4: e1173766. doi:10.1080/23273747.2016.1173766.
- ^ a b Snyder NF, Meretsky VJ (2002). "California Condors and DDE: A re-evaluation". Ibis. 145 (1): 136–151. doi:10.1046/j.1474-919X.2003.00132.x.
- ^ "The Case of DDT: Revisiting the Impairment". US Environmental Protection Agency (EPA). Archived from the original on August 5, 2021. Retrieved August 5, 2021.
- ^ Smith, Jeff (May 9, 2017). "The Science Behind Northport's Gull Island". MyNorth.com. Archived from the original on May 19, 2021. Retrieved September 8, 2020.
- ^ "Endangered and Threatened Wildlife and Plants; 12-Month Petition Finding and Proposed Rule To Remove the Brown Pelican (Pelecanus occidentalis) From the Federal List of Endangered and Threatened Wildlife; Proposed Rule", Fish and Wildlife Service, U.S. Department of the Interior, February 20, 2008. 73 FR 9407
- ^ Moir J (November 15, 2010). "New Hurdle for California Condors May Be DDT From Years Ago". The New York Times. Archived from the original on September 21, 2017. Retrieved February 7, 2017.
- ^ Kurle CM, Bakker VJ, Copeland H, Burnett J, Jones Scherbinski J, Brandt J, Finkelstein ME (2016). "Terrestrial Scavenging of Marine Mammals: Cross-Ecosystem Contaminant Transfer and Potential Risks to Endangered California Condors (Gymnogyps californianus)". Environmental Science & Technology. 50 (17): 9114–9123. Bibcode:2016EnST...50.9114K. doi:10.1021/acs.est.6b01990. PMID 27434394. S2CID 206559840. Archived from the original on June 22, 2020. Retrieved June 13, 2019.
- ^ Walker CH, Sibly RM, Hopkin SP, Peakall DB (2006). Principles of ecotoxicology (3rd ed.). Boca Raton, FL: CRC/Taylor & Francis. pp. 300 ff. ISBN 978-0-8493-3635-5. Archived from the original on July 31, 2020. Retrieved August 29, 2022.
- ^ Guillette LJ (2006). "Endocrine Disrupting Contaminants" (PDF). Archived from the original (PDF) on March 25, 2009. Retrieved February 2, 2007.
- ^ Holm L, Blomqvist A, Brandt I, Brunström B, Ridderstråle Y, Berg C (October 2006). "Embryonic exposure to o,p'-DDT causes eggshell thinning and altered shell gland carbonic anhydrase expression in the domestic hen". Environmental Toxicology and Chemistry. 25 (10): 2787–2793. doi:10.1897/05-619R.1. PMID 17022422. S2CID 9298213.
- ^ "Endocrine (Hormone) Disruptors". United States Fish and Wildlife Service. Archived from the original on October 8, 2015. Retrieved April 8, 2015.
- ^ "Endocrine Disruptors" (PDF). National Institute of Environmental Health Sciences. 2007. Archived from the original (PDF) on March 5, 2016. Retrieved April 8, 2015 – via George Washington Birthplace National Monument.
- ^ "European Food Safety Administration – DDT" (PDF). Archived from the original (PDF) on June 3, 2018. Retrieved October 29, 2014.
- ^ a b "DDT" (PDF). National Toxicology Program. Archived (PDF) from the original on May 22, 2016. Retrieved October 29, 2014.
- ^ IARC – DDT (PDF). 1992. ISBN 9789283212539. Archived (PDF) from the original on October 29, 2014. Retrieved October 29, 2014.
- ^ Kelce, William R.; Stone, Christy R.; Laws, Susan C.; Gray, L. Earl; Kemppainen, Jon A.; Wilson, Elizabeth M. (1995). "Persistent DDT metabolite p,p'–DDE is a potent androgen receptor antagonist". Nature. 375 (6532): 581–585. Bibcode:1995Natur.375..581K. doi:10.1038/375581a0. ISSN 0028-0836. PMID 7791873. S2CID 4344932. Archived from the original on November 17, 2020. Retrieved October 14, 2020.
- ^ a b Cohn BA, Wolff MS, Cirillo PM, Sholtz RI (October 2007). "DDT and breast cancer in young women: new data on the significance of age at exposure". Environmental Health Perspectives. 115 (10): 1406–1414. Bibcode:2007EnvHP.115.1406C. doi:10.1289/ehp.10260. PMC 2022666. PMID 17938728.
- ^ World Health Organization, The WHO Recommended Classification of Pesticides by Hazard Archived July 4, 2021, at the Wayback Machine, 2005.
- ^ a b c Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S (June 2012). "The effects of oxidative stress on female reproduction: a review". Reproductive Biology and Endocrinology. 10 (1): 49. doi:10.1186/1477-7827-10-49. PMC 3527168. PMID 22748101.
In general, incidental human exposure to DDT has been considered relatively non-toxic, but prolonged exposure has long been recognized to adversely affect reproduction.
- ^ Jurewicz J, Hanke W, Radwan M, Bonde JP (January 2010). "Environmental factors and semen quality". International Journal of Occupational Medicine and Environmental Health. 22 (4): 305–329. doi:10.2478/v10001-009-0036-1 (inactive November 11, 2024). PMID 20053623. S2CID 6681999.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Eskenazi B, Chevrier J, Rosas LG, Anderson HA, Bornman MS, Bouwman H, Chen A, Cohn BA, de Jager C, Henshel DS, Leipzig F, Leipzig JS, Lorenz EC, Snedeker SM, Stapleton D (September 2009). "The Pine River statement: human health consequences of DDT use". Environmental Health Perspectives. 117 (9): 1359–1367. Bibcode:2009EnvHP.117.1359E. doi:10.1289/ehp.11748. PMC 2737010. PMID 19750098.
Overall, the few studies conducted to date suggest that DDT exposure may affect time to pregnancy, but more research is needed.
- ^ Chevrier J, Eskenazi B, Holland N, Bradman A, Barr DB (August 2008). "Effects of exposure to polychlorinated biphenyls and organochlorine pesticides on thyroid function during pregnancy". American Journal of Epidemiology. 168 (3): 298–310. doi:10.1093/aje/kwn136. PMC 2727265. PMID 18550560.
- ^ Reardon, Sara (August 16, 2018). "Autism and DDT: What one million pregnancies can – and can't – reveal". Nature. doi:10.1038/d41586-018-05994-1. ISSN 0028-0836. S2CID 81471566. Archived from the original on August 19, 2018. Retrieved August 17, 2018.
- ^ Brown AS, Cheslack-Postava K, Rantakokko P, Kiviranta H, Hinkka-Yli-Salomäki S, McKeague IW, Surcel HM, Sourander A (November 2018). "Association of Maternal Insecticide Levels With Autism in Offspring From a National Birth Cohort". The American Journal of Psychiatry. 175 (11): 1094–1101. doi:10.1176/appi.ajp.2018.17101129. PMC 6377859. PMID 30111184.
- ^ "IARC Monographs evaluate DDT, lindane, and 2,4-D" (PDF). Archived (PDF) from the original on April 13, 2020. Retrieved August 13, 2015.
- ^ VoPham T, Bertrand KA, Hart JE, Laden F, Brooks MM, Yuan JM, Talbott EO, Ruddell D, Chang CH, Weissfeld JL (March 2017). "Pesticide exposure and liver cancer: a review". Cancer Causes & Control. 28 (3): 177–190. doi:10.1007/s10552-017-0854-6. PMC 5336347. PMID 28194594.
- ^ Park JH, Cha ES, Ko Y, Hwang MS, Hong JH, Lee WJ (April 2014). "Exposure to Dichlorodiphenyltrichloroethane and the Risk of Breast Cancer: A Systematic Review and Meta-analysis". Osong Public Health and Research Perspectives. 5 (2): 77–84. doi:10.1016/j.phrp.2014.02.001. PMC 4064641. PMID 24955316.
- ^ Ingber SZ, Buser MC, Pohl HR, Abadin HG, Murray HE, Scinicariello F (December 2013). "DDT/DDE and breast cancer: a meta-analysis". Regulatory Toxicology and Pharmacology. 67 (3): 421–433. doi:10.1016/j.yrtph.2013.08.021. PMC 11298241. PMID 24021539.
- ^ Smith-Bindman R (July 2012). "Environmental causes of breast cancer and radiation from medical imaging: findings from the Institute of Medicine report". Archives of Internal Medicine. 172 (13): 1023–1027. doi:10.1001/archinternmed.2012.2329. PMC 3936791. PMID 22688684.
- ^ Clapp RW, Jacobs MM, Loechler EL (2008). "Environmental and occupational causes of cancer: new evidence 2005–2007". Reviews on Environmental Health. 23 (1): 1–37. doi:10.1515/REVEH.2008.23.1.1. PMC 2791455. PMID 18557596.
- ^ Cohn BA, La Merrill M, Krigbaum NY, Yeh G, Park JS, Zimmermann L, Cirillo PM (August 2015). "DDT Exposure in Utero and Breast Cancer". The Journal of Clinical Endocrinology and Metabolism. 100 (8): 2865–2872. doi:10.1210/jc.2015-1841. PMC 4524999. PMID 26079774.
- ^ "Malaria Fact sheet N°94". WHO. Archived from the original on September 3, 2014. Retrieved February 2, 2016.
- ^ a b c Weir K (June 29, 2007). "Rachel Carson's birthday bashing". Salon.com. Archived from the original on April 15, 2008. Retrieved July 1, 2007.
- ^ Paull J (November 3, 2007). "Toxic Colonialism". New Scientist. 196 (2628): 25. doi:10.1016/S0262-4079(07)62774-2. Archived from the original on April 24, 2015. Retrieved August 26, 2017.
- ^ "World Malaria Report" (PDF). World Health Organization. 2009. Archived (PDF) from the original on January 12, 2010. Retrieved December 17, 2009.
- ^ "WHO Urges Use of DDT in Africa". Washington Post. September 16, 2006. Archived from the original on September 2, 2017. Retrieved August 22, 2017.
- ^ "Widespread use of DDT for malaria control worries environmentalist". Africa Renewal. January 5, 2022.
- ^ Garrett, Laurie (1994). The Coming Plague: Newly Emerging Diseases in a World Out of Balance. Farrar, Straus and Giroux. p. 51. ISBN 978-1-4299-5327-6. Archived from the original on October 19, 2021. Retrieved August 29, 2022.
- ^ McNeil DG (December 27, 2010). "Malaria: A Disease Close to Eradication Grows, Aided by Political Tumult in Sri Lanka". The New York Times. Archived from the original on January 4, 2017. Retrieved February 7, 2017.
- ^ Karunaweera ND, Galappaththy GN, Wirth DF (2014). "On the road to eliminate malaria in Sri Lanka: lessons from history, challenges, gaps in knowledge and research needs". Malaria Journal. 13: 59. doi:10.1186/1475-2875-13-59. PMC 3943480. PMID 24548783.
- ^ "Who gives indoor use of DDT a clean bill of health for controlling malaria". World Health Organization. Archived from the original on September 18, 2006.
- ^ "Countries move toward more sustainable ways to roll back malaria". World Health Organization. Archived from the original on May 6, 2009.
- ^ Yamey G (May 2004). "Roll Back Malaria: a failing global health campaign". BMJ. 328 (7448): 1086–1087. doi:10.1136/bmj.328.7448.1086. PMC 406307. PMID 15130956.
- ^ Griffing SM, Gamboa D, Udhayakumar V (2013). "The history of 20th century malaria control in Peru". Malaria Journal. 12: 303. doi:10.1186/1475-2875-12-303. PMC 3766208. PMID 24001096.
- ^ Curtis CF (December 2002). "Should the use of DDT be revived for malaria vector control?". Biomédica. 22 (4): 455–461. doi:10.7705/biomedica.v22i4.1171. PMID 12596442.
- ^ a b c d e f Curtis CF (February 1996). "Control of Malaria Vectors in Africa and Asia". University of Minnesota. Archived from the original on October 2, 2007.
- ^ Sharma VP (September 1999). "Current scenario of malaria in India". Parassitologia. 41 (1–3): 349–353. PMID 10697882.
- ^ Agarwal R (May 2001). "No Future in DDT: A case study of India". Pesticide Safety News.
- ^ a b Sharma SN, Shukla RP, Raghavendra K, Subbarao SK (June 2005). "Impact of DDT spraying on malaria transmission in Bareilly District, Uttar Pradesh, India". Journal of Vector Borne Diseases. 42 (2): 54–60. PMID 16161701.
- ^ Hargreaves K, Hunt RH, Brooke BD, Mthembu J, Weeto MM, Awolola TS, Coetzee M (December 2003). "Anopheles arabiensis and An. quadriannulatus resistance to DDT in South Africa". Medical and Veterinary Entomology. 17 (4): 417–422. doi:10.1111/j.1365-2915.2003.00460.x. PMID 14651656. S2CID 22748077.
- ^ a b Grieco JP, Achee NL, Chareonviriyaphap T, Suwonkerd W, Chauhan K, Sardelis MR, Roberts DR (2007). Krishna S (ed.). "A new classification system for the actions of IRS chemicals traditionally used for malaria control". PLOS ONE. 2 (8): e716. Bibcode:2007PLoSO...2..716G. doi:10.1371/journal.pone.0000716. PMC 1934935. PMID 17684562.
- ^ a b c Mabaso ML, Sharp B, Lengeler C (August 2004). "Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying". Tropical Medicine & International Health. 9 (8): 846–856. doi:10.1111/j.1365-3156.2004.01263.x. PMID 15303988. S2CID 10018052.
- ^ Sharma VP (December 2003). "DDT: The fallen angel" (PDF). Current Science. 85 (11): 1532–1537. Archived from the original (PDF) on May 18, 2005.
- ^ "In Malaria War, South Africa Turns To Pesticide Long Banned in the West" Archived October 13, 2007, at the Wayback Machine, Roger Thurow, The Wall Street Journal, July 26, 2001
- ^ Science Daily (May 9, 2009). "Unprecedented Use Of DDT Concerns Experts". ScienceDaily.com. Archived from the original on May 14, 2009. Retrieved May 30, 2009.
- ^ Jayashree J (June 10, 2009). "Pesticide level in veggies, fruits rises". The Economic Times. Archived from the original on July 11, 2018. Retrieved June 10, 2009.
- ^ Sanjana (June 13, 2009). "A Whole Fruit". Tehelka. 6 (23). Archived from the original on April 5, 2012. Retrieved June 8, 2009.
- ^ Chakravartty A (June 8, 2009). "State public libraries gasp for breath". Indian Express. Archived from the original on April 13, 2020. Retrieved June 8, 2009.
- ^ Katima J (June 2009). "African NGOs outline commitment to malaria control without DDT" (PDF). Pesticides News (84): 5. Archived from the original (PDF) on February 24, 2016.
- ^ Ghana News Agency (November 17, 2009). "Ministry moves to check unorthodox fishing methods". Ghana News Agency. Archived from the original on January 18, 2012. Retrieved November 18, 2009.
- ^ Appiah S (April 27, 2010). "Northern fisherfolks complain of committee's harassment". Joy Online. Archived from the original on April 29, 2010. Retrieved April 27, 2010.
- ^ Souder W (September 4, 2012). "Rachel Carson Didn't Kill Millions of Africans". Slate. Archived from the original on April 22, 2014. Retrieved September 5, 2012.
- ^ Oreskes, Naomi; Conway, Erik M. (2010). Merchants of Doubt. New York: Bloomsbury. p. 217. ISBN 978-1-59691-610-4.
- ^ Baum, Rudy M. (June 4, 2007). "Rachel Carson". Chemical and Engineering News. 85 (23): 5.
- ^ Palmer, Michael (September 29, 2016). "The ban of DDT did not cause millions to die from malaria" (PDF). University of Waterloo. Archived (PDF) from the original on June 16, 2021. Retrieved August 14, 2018.
- ^ Herren HR, Mbogo C (July 2010). "The role of DDT in malaria control". Environmental Health Perspectives. 118 (7): A282–A283, author reply A283. doi:10.1289/ehp.1002279. PMC 2920925. PMID 20601331.
- ^ Quiggin J, Lambert T (May 2008). "Rehabilitating Carson". Prospect. Archived from the original on April 13, 2020. Retrieved June 22, 2012.
- ^ Sarvana A (May 28, 2009). "Bate and Switch: How a free-market magician manipulated two decades of environmental science". Natural Resources New Service. Archived from the original on May 24, 2010. Retrieved June 2, 2009.
- ^ Gutstein D (2009). Not a Conspiracy Theory: How Business Propaganda is Hijacking Democracy. Key Porter Books. ISBN 978-1-55470-191-9. Archived from the original on October 19, 2021. Retrieved August 29, 2022.. Relevant excerpt at Gutstein D (January 22, 2010). "Inside the DDT Propaganda Machine". The Tyee. Archived from the original on January 25, 2010. Retrieved January 22, 2010.
- ^ "Indoor Residual Spraying" (PDF). World Health Organization. 2019. Archived (PDF) from the original on July 29, 2020. Retrieved March 14, 2019.
- ^ Zhu, Xiaolong; Hu, Chunhua T.; Yang, Jingxiang; Joyce, Leo A.; Qiu, Mengdi; Ward, Michael D.; Kahr, Bart (October 11, 2019). "Manipulating Solid Forms of Contact Insecticides for Infectious Disease Prevention". Journal of the American Chemical Society. 141 (42): 16858–16864. Bibcode:2019JAChS.14116858Z. doi:10.1021/jacs.9b08125. PMID 31601104. S2CID 204244148.
- ^ Chang, Kenneth (October 17, 2019). "A Nazi Version of DDT Was Forgotten. Could It Help Fight Malaria?". The New York Times. Archived from the original on January 1, 2022. Retrieved October 18, 2019.
- ^ Killeen GF, Fillinger U, Kiche I, Gouagna LC, Knols BG (October 2002). "Eradication of Anopheles gambiae from Brazil: lessons for malaria control in Africa?". The Lancet. Infectious Diseases. 2 (10): 618–627. doi:10.1016/S1473-3099(02)00397-3. PMID 12383612.
- ^ "Malaria Worldwide – How Can Malaria Cases and Deaths Be Reduced? – Larval Control and Other Vector Control Interventions". CDC.gov. January 29, 2019. Archived from the original on July 9, 2017. Retrieved September 9, 2017.
- ^ "Impact of long-lasting insecticidal-treated nets (LLINs) and artemisinin-based combination therapies (ACTs) measured using surveillance data in four African countries". World Health Organization, January 31, 2008.
- ^ Malaria deaths halved in Rwanda and Ethiopia Better drugs, mosquito nets are the crucial tools Archived February 10, 2008, at the Wayback Machine, David Brown (Washington Post), SF Chronicle, A-12, February 1, 2008.
- ^ "World Health Organization, A story to be shared: The successful fight against malaria in Vietnam", November 6, 2000. Archived February 26, 2008, at the Wayback Machine
- ^ a b "DDT & Malaria" (PDF). Archived from the original (PDF) on July 26, 2011. Retrieved March 11, 2009.
- ^ Goodman CA, Mills AJ (December 1999). "The evidence base on the cost-effectiveness of malaria control measures in Africa". Health Policy and Planning. 14 (4): 301–312. doi:10.1093/heapol/14.4.301. PMID 10787646.
- ^ Kamolratanakul P, Butraporn P, Prasittisuk M, Prasittisuk C, Indaratna K (October 2001). "Cost-effectiveness and sustainability of lambdacyhalothrin-treated mosquito nets in comparison to DDT spraying for malaria control in western Thailand". The American Journal of Tropical Medicine and Hygiene. 65 (4): 279–284. doi:10.4269/ajtmh.2001.65.279. PMID 11693869.
- ^ Goodman CA, Mnzava AE, Dlamini SS, Sharp BL, Mthembu DJ, Gumede JK (April 2001). "Comparison of the cost and cost-effectiveness of insecticide-treated bednets and residual house-spraying in KwaZulu-Natal, South Africa". Tropical Medicine & International Health. 6 (4): 280–295. doi:10.1046/j.1365-3156.2001.00700.x. PMID 11348519. S2CID 28103584.
- ^ Corin SE, Weaver SA (2005). "A risk analysis model with an ecological perspective on DDT and malaria control in South Africa" (PDF). Journal of Rural and Tropical Public Health. 4 (4): 21–32. Archived (PDF) from the original on March 6, 2006. Retrieved January 30, 2006.
- ^ Over M, Bakote'e B, Velayudhan R, Wilikai P, Graves PM (August 2004). "Impregnated nets or DDT residual spraying? Field effectiveness of malaria prevention techniques in solomon islands, 1993–1999". The American Journal of Tropical Medicine and Hygiene. 71 (2 Suppl): 214–223. doi:10.4269/ajtmh.2004.71.214. PMID 15331840.
- ^ Barat LM (January 2006). "Four malaria success stories: how malaria burden was successfully reduced in Brazil, Eritrea, India, and Vietnam". The American Journal of Tropical Medicine and Hygiene. 74 (1): 12–16. doi:10.4269/ajtmh.2006.74.12. PMID 16407339.
- ^ Mint Mohamed Lemine A, Ould Lemrabott MA, Niang EH, Basco LK, Bogreau H, Faye O, Ould Mohamed Salem Boukhary A (June 2018). "Pyrethroid resistance in the major malaria vector Anopheles arabiensis in Nouakchott, Mauritania". Parasites & Vectors. 11 (1): 344. doi:10.1186/s13071-018-2923-4. PMC 5998517. PMID 29895314.
Further reading
[edit]- Berry-Cabán, Cristóbal S. "DDT and silent spring: fifty years after". Journal of Military and Veterans' Health 19 (2011): 19–24. online
- Conis, Elena. "Debating the health effects of DDT: Thomas Jukes, Charles Wurster, and the fate of an environmental pollutant". Public Health Reports 125.2 (2010): 337–342. online
- Davis, Frederick Rowe. "Pesticides and the perils of synecdoche in the history of science and environmental history". History of Science 57.4 (2019): 469–492. doi:10.1177/0073275319848964
- "DDT Banning" in Richard L. Wilson, ed. Historical Encyclopedia of American Business, Vol I. Accounting Industry – Google, (Salem Press: 2009) p. 223 ISBN 978-1587655180. OCLC 430057855
- Dunlap, Thomas, ed. DDT, Silent Spring, and the Rise of Environmentalism (University of Washington Press, 2008). OCLC 277748763
- Dunlap, Thomas, ed. DDT, Silent Spring, and the Rise of Environmentalism: Classic texts (University of Washington Press, 2015). ISBN 978-0295998947. OCLC 921868876
- Jarman Walter M., Ballschmiter Karlheinz (2012). "From coal to DDT: the history of the development of the pesticide DDT from synthetic dyes till Silent Spring". Endeavour. 36 (4): 131–142. doi:10.1016/j.endeavour.2012.10.003. PMID 23177325.
- Kinkela, David. DDT and the American Century: Global Health, Environmental Politics, and the Pesticide That Changed the World (University of North Carolina Press, 2011). ISBN 978-0807835098. OCLC 934360239
- Morris, Peter J. T. (2019). "Chapter 9: A Tale of Two Nations: DDT in the United States and the United Kingdom". Hazardous Chemicals: Agents of Risk and Change, 1800–2000. Environment in History: International Perspectives 17. Berghahn Books. 294–327. doi:10.2307/j.ctv1850hst.15 (book: doi:10.2307/j.ctv1850hst; JSTOR j.ctv1850hst).
External links
[edit]This section's use of external links may not follow Wikipedia's policies or guidelines. (April 2023) |
- Chemistry
- DDT at The Periodic Table of Videos (University of Nottingham)
- Toxicity
- "DDT Technical Fact Sheet" (PDF). National Pesticide Information Center. Archived (PDF) from the original on June 29, 2003.
- "DDT General Fact Sheet" (PDF). National Pesticide Information Center. Archived (PDF) from the original on March 22, 2003.
- Scorecard: The Pollution Information Site – DDT
- Interview with Barbara Cohn, PhD about DDT and breast cancer
- Pesticide residues in food 2000 : DDT
- Politics and DDT
- Swartz, Aaron (September–October 2007). "Rachel Carson, Mass Murderer?: The creation of an anti-environmental myth". Extra!.
- Tierney, John (June 5, 2007). "Fateful Voice of a Generation Still Drowns Out Real Science". The New York Times.
- Malaria and DDT
- Berenbaum M (June 4, 2005). "If Malaria's the Problem, DDT's Not the Only Answer". Washington Post.
- 'Andrew Spielman, Harvard School of Public Health, discusses environmentally friendly control of Malaria and uses of DDT Freeview video provided by the Vega Science Trust
- "Ugandan farmers push for DDT ban". ABC News. Australian Broadcasting Commission. May 31, 2008.
- DDT in popular culture
- Dunning, Brian (November 2, 2010). "Skeptoid #230: DDT: Secret Life of a Pesticide". Skeptoid.
- Phil Allegretti Pesticide Collection consisting of ephemera and 3-D objects, including cans, sprayers, and diffusers, related to DDT pesticide and insecticide in the United States in the mid-20th century (all images freely available for download in variety of formats from Science History Institute Digital Collections at digital.sciencehistory.org).