The pharmacology of progesterone, a progestogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.[1][2]
Progesterone is a naturally occurring and bioidentical progestogen, or an agonist of the progesterone receptor, the biological target of progestogens like endogenous progesterone.[1] Progesterone also has antimineralocorticoid and inhibitory neurosteroid activity, whereas it appears to have little or no glucocorticoid or antiandrogenic activity and has no androgenic activity.[1] Because of its progestogenic activity, progesterone has functional antiestrogenic effects in certain tissues such as the uterus, cervix, and vagina.[1] In addition, progesterone has antigonadotropic effects due to its progestogenic activity and can inhibit fertility and suppress sex hormone production.[1] Progesterone differs from progestins (synthetic progestogens) like medroxyprogesterone acetate and norethisterone, with implications for pharmacodynamics and pharmacokinetics as well as efficacy, tolerability, and safety.[1]
Progesterone can be taken by mouth, in through the vagina, and by injection into muscle or fat, among other routes.[1] A progesterone vaginal ring and progesterone intrauterine device are also available as pharmaceutical products.[3][4]
Mechanism of action
[edit]Progesterone is a progestogen, or an agonist of the nuclear progesterone receptors (PRs), the PR-A, PR-B, and PR-C.[1] In one study, progesterone showed EC50 values of 7.7 nM for the human PR-A and 8.0 nM for the human PR-B.[5] In addition to the PRs, progesterone is an agonist of the membrane progesterone receptors (mPRs), including the mPRα, mPRβ, mPRγ, mPRδ, and mPRϵ.[6][7] It is also a potent antimineralocorticoid (antagonist of the mineralocorticoid receptor (MR)),[8][9] as well as a very weak glucocorticoid (agonist of the glucocorticoid receptor).[10][11] Progesterone does not interact significantly with the androgen receptor (AR) or with the estrogen receptor (ER).[1] In addition to its activity as a steroid hormone, progesterone is a neurosteroid.[12] Specifically, it is an antagonist of the sigma σ1 receptor,[13][14] a negative allosteric modulator of nicotinic acetylcholine receptors,[12] and, via its active metabolites allopregnanolone and pregnanolone, a potent positive allosteric modulator of the GABAA receptor, the major signaling receptor of the inhibitory neurotransmitter γ-aminobutyric acid (GABA).[15]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|
| Progesterone | 50 | 0 | 0 | 10 | 100 | 0 | 36 |
| Notes: Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. Sources: [1] | |||||||
| Compound | Form | Dose for specific uses (mg)[c] | DOA[d] | |||
|---|---|---|---|---|---|---|
| TFD[e] | POICD[f] | CICD[g] | ||||
| Algestone acetophenide | Oil soln. | – | – | 75–150 | 14–32 d | |
| Gestonorone caproate | Oil soln. | 25–50 | – | – | 8–13 d | |
| Hydroxyprogest. acetate[h] | Aq. susp. | 350 | – | – | 9–16 d | |
| Hydroxyprogest. caproate | Oil soln. | 250–500[i] | – | 250–500 | 5–21 d | |
| Medroxyprog. acetate | Aq. susp. | 50–100 | 150 | 25 | 14–50+ d | |
| Megestrol acetate | Aq. susp. | – | – | 25 | >14 d | |
| Norethisterone enanthate | Oil soln. | 100–200 | 200 | 50 | 11–52 d | |
| Progesterone | Oil soln. | 200[i] | – | – | 2–6 d | |
| Aq. soln. | ? | – | – | 1–2 d | ||
| Aq. susp. | 50–200 | – | – | 7–14 d | ||
|
Notes and sources:
| ||||||
Antimineralocorticoid activity
[edit]Progesterone is a potent antimineralocorticoid.[8][9][35] It has 1000% of the affinity of aldosterone, the major endogenous agonist, for the human MR, and 100% of the affinity of aldosterone for the rat MR.[36][1][8] Progesterone produces antimineralocorticoid effects such as natriuresis (excretion of sodium in the urine) at normal physiological concentrations.[9] A 200 mg dose of oral progesterone is considered to be approximately equivalent in antimineralocorticoid effect to a 25 to 50 mg dose of the potent antimineralocorticoid spironolactone, which itself is a derivative of progesterone.[37] Doses of progesterone of 50 to 200 mg by intramuscular injection, which are similar to progesterone exposure in the third trimester of pregnancy, have also been reported to produce antimineralocorticoid-like effects.[35] The antimineralocorticoid effects of progesterone underlie its ability to lower blood pressure and reduce water and salt retention and its potential application in the treatment of hypertension.[38][1][39][35] An active metabolite of progesterone, 11-deoxycorticosterone (21-hydroxyprogesterone), is a precursor of aldosterone and has strong mineralocorticoid activity (i.e., is a strong agonist of the MR).[37] However, it is formed in relatively small amounts, and any such effects produced by it are usually outweighed by the antimineralocorticoid activity of progesterone.[37] Progesterone may be a relatively weak antimineralocorticoid in vivo.[40]
Glucocorticoid activity
[edit]Progesterone is a partial agonist of the glucocorticoid receptor (GR).[1][10][11][41][42] It has about 35% of the affinity of dexamethasone, a corticosteroid, for the human GR, and about 3 to 11% of the affinity of dexamethasone for the rat GR.[36] However, progesterone appears to show weak or no glucocorticoid activity and no antiglucocorticoid activity in vitro and in animals.[42] Nonetheless, progesterone has been found to upregulate the thrombin receptor in vascular smooth muscle cells in vitro, a glucocorticoid effect, and this could have clinical relevance in relation to risk of blood clots.[1][43]
| Steroid | Class | TR (↑)a | GR (%)b |
|---|---|---|---|
| Dexamethasone | Corticosteroid | ++ | 100 |
| Ethinylestradiol | Estrogen | – | 0 |
| Etonogestrel | Progestin | + | 14 |
| Gestodene | Progestin | + | 27 |
| Levonorgestrel | Progestin | – | 1 |
| Medroxyprogesterone acetate | Progestin | + | 29 |
| Norethisterone | Progestin | – | 0 |
| Norgestimate | Progestin | – | 1 |
| Progesterone | Progestogen | + | 10 |
| Footnotes: a = Thrombin receptor (TR) upregulation (↑) in vascular smooth muscle cells (VSMCs). b = RBA (%) for the glucocorticoid receptor (GR). Strength: – = No effect. + = Pronounced effect. ++ = Strong effect. Sources: [44] | |||
Androgenic and antiandrogenic activity
[edit]The binding and activity of progesterone at the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT) in the body, is controversial.[45] Some studies have found progesterone to bind to the AR, with agonistic and antagonistic activity exerted, whereas other studies have found very low or no affinity for the AR at all.[45] In animal studies, no androgenic effects have been observed, but weak antiandrogenic effects have been reported.[45] The weak antiandrogenic activity has been attributed not to antagonism of the AR by progesterone, but rather to its weak 5α-reductase inhibition and consequent inhibition of the conversion of testosterone into the more potent DHT.[45] There is no clinical evidence of AR-mediated androgenic or antiandrogenic activity with progesterone.[45] Progesterone has not been associated with any classical androgenic effects in clinical studies in women, including no changes in the blood lipid profile or sex hormone-binding globulin levels, acne, oily skin, hirsutism, or voice deepening, nor with virilization of female fetuses.[46][47][48][49][50] As such, the scientific consensus is that progesterone is clinically neither androgenic nor antiandrogenic.[1][51][52] This is in contrast to many progestins, such as 19-nortestosterone derivatives (e.g., norethisterone, levonorgestrel, dienogest) and 17α-hydroxyprogesterone derivatives (e.g., cyproterone acetate, medroxyprogesterone acetate), which do bind to the AR and have been associated with significant androgenic or antiandrogenic effects depending on the progestin in question.[1][52] Due to its lack of androgenic and antiandrogenic activity, and hence lack of masculinizing and feminizing effects, progesterone is one of the few progestogens that is suitable for use during pregnancy in women at risk for preterm birth or recurrent miscarriage.[53][54]
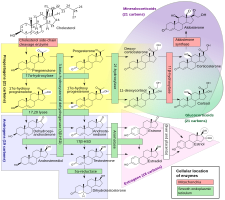
Although progesterone does not have significant AR-mediated androgenic or antiandrogenic activity, it is a precursor and intermediate, albeit distant, in the biosynthesis of androgens from cholesterol.[55][56] For this reason, there has been some speculation that exogenous progesterone could be transformed into androgens by certain tissues that express the requisite enzymes.[56][57] Progesterone is converted by 17α-hydroxylase into 17α-hydroxyprogesterone; 17α-hydroxyprogesterone is converted by 17,20-lyase into androstenedione; and androstenedione is converted by 17β-hydroxysteroid dehydrogenases into testosterone.[55] CYP17A1, the cytochrome P450 gene that encodes 17α-hydroxylase and 17,20-lyase, is expressed mainly in the gonads (ovaries and testes) and the adrenal glands.[58] However, while it is theoretically possible that progesterone could be transformed in the body into androgens, no androgenic effects have been observed in animal studies.[45] In addition, clinical studies, in which women were treated with 100 to 300 mg/day oral progesterone, have found no or only a small increase in levels of 17α-hydroxyprogesterone, and no change in androgen levels, including of dehydroepiandrosterone, androstenedione, and testosterone.[50][59][46] In these studies, levels of estradiol and cortisol, which progesterone is also a precursor of, did not change either, although levels of 11-deoxycorticosterone increased significantly.[59][46] Levels of androgens, like testosterone and dihydrotestosterone (DHT), also do not increase going from the follicular phase to the luteal phase of the menstrual cycle in premenopausal women (progesterone levels being high in the luteal phase).[60]
5α-Reductase inhibition
[edit]Progesterone is a substrate for 5α-reductase, and has been found to act as a competitive inhibitor of this enzyme in vitro in a variety of studies.[1] In one study, it showed IC50 values of 1,375 nM and 88 nM (in the presence of 50 nM androstenedione as the substrate) for 5α-reductase types 1 and 2, respectively.[61] 5α-Reductase is highly expressed in skin, hair follicles, and prostate gland, and is responsible for the transformation of testosterone into the several-fold more potent androgen DHT in such tissues.[62][63] As such, it has been suggested that progesterone might possess some antiandrogenic effect via acting as a 5α-reductase inhibitor.[1] However, inhibition of 5α-reductase by progesterone is described as a weak effect that has only been demonstrated in vitro and at supraphysiological concentrations.[64][65] In accordance, physiological levels of circulating progesterone have not been found to importantly influence circulating DHT concentrations.[60][66]
Congenital 5α-reductase 2 deficiency is a rare intersex condition which is associated with ambiguous genitalia in male fetuses due to a deficiency in DHT production during genital differentiation.[63] Experimental prenatal exposure to established 5α-reductase inhibitors like finasteride has been found to produce similar feminized genital defects in male animals including rodents and monkeys.[67] In contrast, exogenous administration of progesterone to pregnant rodents and monkeys has resulted in minimal abnormality in either male or female pups.[68][69][70][71] In addition, endogenous progesterone levels naturally increase to extremely high concentrations during pregnancy, yet genital defects do not occur.[72] In accordance, while total concentrations of progesterone in pregnant women at term are around 150 ng/mL (~500 nM), free or unbound and hence bioactive concentrations of progesterone are only about 3 ng/mL (~10 nM) due to the high plasma protein binding of progesterone, and these concentrations are still well below the aforementioned IC50 values for inhibition of 5α-reductase types 1 and 2.[73][74] As with endogenous progesterone during pregnancy, exogenous supplemental progesterone during pregnancy has been found not to increase the risk of hypospadias in male infants.[75]
Although systemic progesterone does not appear to be an effective 5α-reductase inhibitor, topical progesterone may produce potent inhibition of 5α-reductase in the skin due to the very high local concentrations that occur.[76][77] A study found that topical progesterone applied to the pubic area in men inhibited 5α-reductase in the skin in this region by 75%.[77][78] In addition to inhibition of 5α-reductase, progesterone is metabolized by 5α-reductase into 5α-dihydroprogesterone (5α-DHP), a compound reported to have some antagonistic activity at the AR.[78][79] However, this compound appears to have no systemic antiandrogenic activity.[80] In spite of its apparent 5α-reductase inhibition, the effectiveness of topical progesterone in the treatment of pattern hair loss has been poor.[79][81][82]
Other activity
[edit]Certain progestins have been found to stimulate the proliferation of MCF-7 breast cancer cells in vitro, an action that is independent of the classical PRs and is instead mediated via the progesterone receptor membrane component-1 (PGRMC1).[83] Progesterone, nomegestrol acetate, and chlormadinone acetate act neutrally and do not stimulate proliferation, whereas norethisterone, desogestrel, levonorgestrel, and drospirenone strongly stimulate proliferation and medroxyprogesterone acetate, dienogest, and dydrogesterone weakly stimulate proliferation.[83][84] As such, progesterone differs from some but not all progestins in the activity mediating this PGRMC1-dependent effect.[83] It is unclear if these findings may explain the different risks of breast cancer observed with progesterone, dydrogesterone, and other progestins such as medroxyprogesterone acetate and norethisterone in clinical studies.[85]
Effects in the body and brain
[edit]The PRs are expressed widely throughout the body, including in the uterus, cervix, vagina, fallopian tubes, breasts, fat, skin, pituitary gland, hypothalamus, and elsewhere throughout the brain.[1][86] Through activation of the PRs (as well as the mPRs), progesterone has many effects, including the following:[1][86]
- Induces endometrial secretory transformation in preparation for pregnancy (>5 ng/mL)[87]
- Prevents estrogen-induced endometrial hyperplasia and increased endometrial cancer risk
- Maintains pregnancy via effects in endometrium (with withdrawal resulting in miscarriage)
- Reduces amount and fibrosity of cervical mucus and causes cervix to become firmer and more tightly closed[88]
- Controls motility and composition of fluid in the fallopian tubes
- Reduced cornification and maturation of the vaginal lining[89]
- Causes water retention in the breasts resulting in temporary enlargement during the menstrual cycle[90][91]
- Mediates lobuloalveolar development of the breasts necessary for lactation
- Suppresses lactation initiation and triggers lactation upon withdrawal (as with parturition)
- Maintains skin health, integrity, appearance, and hydration and slows the rate of aging of the skin[92][93]
- Modulates brain function, with effects on mood, emotionality, and sexuality, as well as cognition and memory
- Exerts negative feedback on the hypothalamic–pituitary–gonadal axis (HPG axis) by suppressing the secretion of the gonadotropins FSH and LH from the pituitary gland (including the mid-cycle gonadotropin surge), thereby inhibiting gonadal sex hormone production as well as ovulation and fertility (>2 ng/mL)[94]
- Increases basal body temperature (by 0.3–0.6 °C (0.5–1.0 °F) relative to preovulation) via the hypothalamus (>4 ng/mL)[95][96]
- Reduces hot flashes via the hypothalamus[97][98]
- Stimulates respiration via the hypothalamus and/or respiratory center[99][100]
- Influences the risk and/or progression of hormone-sensitive cancers including breast cancer and endometrial cancer
Many of the effects of progesterone require estrogen, as estrogens prime tissues for progesterone by inducing expression of the PRs.[1][86] The PRs are induced in the breasts by estrogens, and for this reason, it is assumed that progestogens cannot mediate breast changes in the absence of estrogens.[101]
Progesterone also lowers blood pressure and reduces water and salt retention among other effects via its antimineralocorticoid activity.[1][39]
Progesterone can produce sedative, hypnotic, anxiolytic, euphoric, cognitive-, memory-, and motor-impairing, anticonvulsant, and even anesthetic effects via formation of sufficiently high concentrations of its neurosteroid metabolites and consequent GABAA receptor potentiation in the brain.[38][102][103][104]
Uterine effects
[edit]Under normal physiological circumstances, progesterone secreted by the corpus luteum during the luteal phase of the menstrual cycle produces endometrial transformation of the estrogen-primed uterus in preparation for implantation and pregnancy.[105] Normal progesterone production during the luteal phase is 25 mg/day on average with a range of 15 to 50 mg/day.[106][71] Progesterone levels during the luteal phase range from 7 ng/mL to 22 ng/mL using liquid chromatography–tandem mass spectrometry (LC–MS/MS) per one source.[107] Sustained progesterone levels of more than 5 ng/mL, perhaps approximately 10 ng/mL, are required for full endometrial transformation.[87][108] Progesterone levels of more than 10 ng/mL are rarely associated with luteal-phase defect on the basis of endometrial biopsy.[109]
Luteal-phase levels of progesterone are said to be produced by 25 mg/day progesterone in oil solution by intramuscular injection or by 100 mg/day progesterone by vaginal or rectal administration.[71][110] Progesterone by intramuscular injection in oil solution has been found to produce endometrial transformation at a dose of 10 or 20 mg/day for 14 days (total dose per cycle of 200 mg), whereas a single intramuscular injection of 200 mg progesterone in microcrystalline aqueous suspension provides endometrial transformation after 10 to 14 days.[111] A study found full and equivalent endometrial transformation with subcutaneous injection of 25 mg/day versus 50 mg/day progesterone in aqueous solution.[112] Due to a uterine first-pass effect and markedly higher uterine progesterone levels than with other routes, 45 mg/day vaginal progesterone, a dosage that achieves circulating progesterone levels of only 1 to 5 ng/mL, provides complete endometrial transformation.[113][110] Conversely, intranasal administration of progesterone achieving progesterone levels of 2 to 5 ng/mL was ineffective.[113] Transdermal progesterone achieves very low progesterone levels and is considered to be ineffective for endometrial protection.[113][114][115]
The endometrial transformation dosage of oral micronized progesterone in women has been listed as 200 to 300 mg/day or 4,200 mg total per cycle.[116][1] However, a clinical study found that 300 mg/day oral micronized progesterone was insufficient for full endometrial transformation.[117] Similarly, 600 to 1,000 mg/day oral micronized progesterone has been reported to be ineffective for achieving complete endometrial transformation.[113][112] Despite inadequate endometrial transformation with oral progesterone, continuous 100 mg/day oral micronized progesterone or cyclic 200 mg/day oral micronized progesterone is effective for protection of the endometrium against estrogen-induced endometrial hyperplasia.[118] On the other hand, and in contrast to progestins, typical clinical doses of oral micronized progesterone have been associated with failure to prevent increased endometrial cancer risk caused by estrogen therapy.[119]
Antiestrogenic effects
[edit]Progesterone, like all progestogens, has antiestrogenic effects in certain tissues such as the uterus, cervix, vagina, and breasts, and possibly also the brain.[1][120][121] These effects are mediated by activation of the PR in these tissues.[1] Progesterone does not have antiestrogenic effects in the more conventional sense of binding to and antagonizing the ER or binding to and inhibiting enzymes involved in estrogen biosynthesis.[1] Instead, for instance in the endometrium, progesterone causes downregulation of the ER and upregulation of the estrogen-inactivating enzymes 17β-hydroxysteroid dehydrogenase 2 (converts estradiol into estrone) and estrone sulfotransferase (converts estrone into estrone sulfate).[1] The antiestrogenic effects of progesterone and other progestogens form the basis for their only approved indication in menopausal hormone therapy: prevention of long-term unopposed estrogen-induced endometrial hyperplasia and increased endometrial cancer risk in women with intact uteruses.[1]
In the breasts, progesterone and other progestogens downregulate the ER as well as the estrogen-activating enzymes steroid sulfatase (converts estrone sulfate into estrone) and 17β-hydroxysteroid-dehydrogenase 1 (converts estrone into estradiol) and upregulates estrone sulfotransferase.[120][121] However, other studies suggest that progestogens do not downregulate ER expression in the breasts.[122][123] When applied directly to the breasts in women, progesterone can block the proliferative effects of estradiol.[124][101][125][126][127][128][129][85] However, the concentrations were supraphysiological and the same may not be the case with more physiological concentrations.[127][85] Cellular proliferation in the breasts is greatest in the luteal phase of the menstrual cycle, when progesterone levels are highest.[85]
It has been hypothesized that progestogens may counteract various effects of estrogens in the brain such as stimulatory and excitatory effects on neuronal activity.[1] Progesterone moreover has a special position among progestogens concerning such actions due to its inhibitory neurosteroid metabolites and their central depressant effects.[1] It has been suggested that these actions of progestogens may explain unfavorable effects on mood that have been reported with these medications in some women.[1] However, the mutual interactions of estrogens and progestogens in the brain in general are controversial and require more research.[1]
Progesterone can produce body-wide antiestrogenic effects at very high doses in both women and men via its antigonadotropic effects and consequent suppression of gonadal estrogen production (see below).[1][130] These antigonadotropic effects are mediated by hyperactivation of the PR.[1][130]
Effects on the HPG axis
[edit]Antigonadotropic effects
[edit]Progestogens have antigonadotropic effects at sufficiently high doses via activation of the PR and consequent negative feedback on and hence suppression of the hypothalamic–pituitary–gonadal axis (HPG axis).[130] This results in suppression of gonadotropin secretion and by extension interference with fertility and gonadal sex hormone production.[130] Progesterone prevents ovulation by suppressing the mid-cycle surge in gonadotropin secretion during the menstrual cycle.[131][94]
The ovulation-inhibiting (i.e., contraceptive) dosage of oral crystalline (non-micronized) progesterone in women is 300 mg/day or greater.[71][132][1][133][134] However, this figure is based on limited clinical data.[71] In the clinical research in the 1950s that determined this dosage, ovulation inhibition occurred in 50 to 100% of women when assessed via measures including urinary pregnanediol excretion, daily basal body temperatures, endometrial biopsies, and vaginal smears.[132][135][136][137][138] Another study found that ovulation inhibition with 300 mg/day oral non-micronized progesterone occurred in a "proportion of the cases" when assessed via laparotomy.[137] A third study found that ovulation was inhibited in only 38% of women treated with 1,000 mg/day oral non-micronized progesterone.[133] A fourth publication stated that even 750 to 1,000 mg/day oral non-micronized progesterone had weak effects as evidenced by poor thermogenic effect, weak endometrial effect, and lack of production of withdrawal bleeding in amenorrheic women.[139][140] Neumann and colleagues listed the ovulation-inhibiting dosage of oral non-micronized progesterone in women as 300 to 500 mg/day or as 400 mg/day but provided no other details.[134][141][142][143]
In a study of a progesterone vaginal ring alone or in combination with estradiol that released 1.5 to 3 mg/day progesterone and achieved mean progesterone levels varying between 0.7 and 1.6 ng/mL (mean 0.9 ng/mL) during anovulatory cycles, ovulation occurred in 18 of 30 (60%) menstrual cycles.[144] A study of a vaginal progesterone ring that released almost 10 mg/day progesterone and maintained mean progesterone levels of 4.4 ng/mL (range 2.4–6.5 ng/mL) found that ovulation was inhibited in some but not all women.[145][146] In another study, a progesterone vaginal ring that released about 10 mg/day progesterone and produced progesterone levels of around 4 ng/mL (range 3–5.2 ng/mL) resulted in ovulation occurring in 25% of treated breastfeeding women compared to a rate of 56% in a control group of breastfeeding women.[147] A study in rhesus monkeys found that a vaginal ring delivering 0.235 or 1.77 mg/day progesterone inhibited ovulation in all monkeys at the higher dose and in a proportion of monkeys at the lower dose.[68][148] A dose of progesterone of 5 to 10 mg/day by intramuscular injection has been found to prevent ovulation in women and has been considered effective as a progestogen-only injectable contraceptive.[149][150][151]
Short-term therapy with 300 mg/day oral progesterone had no effect on luteinizing hormone pulse frequency in women.[152] Treatment with a high dosage of oral progesterone of 100 mg four times per day (or 400 mg/day total) in men for 10 days did not cause any change in testosterone levels, suggesting that oral progesterone has little or no antigonadotropic effect in males at typical clinical dosages.[38][153] In addition, a study found that administration of 1,000 mg/day oral progesterone for 3 months had no significant effect on urinary gonadotropin excretion.[71] On the other hand, a single 50 mg intramuscular injection of progesterone, which is associated with high progesterone levels of approximately 50 ng/mL (or early- to mid-pregnancy levels),[154][155][156] resulted in substantial (50–60%) suppression of luteinizing hormone, follicle-stimulating hormone, and testosterone levels in men.[157][158] Similarly, continuous or intermittent intravenous injections of 100 to 400 mg/day progesterone for 10 days significantly decreased urinary gonadotropin excretion.[71][159] Progestogens in general are able to suppress gonadal testosterone production in men by a maximum of about 70 to 80% or to just above castrate levels when used at sufficiently high doses.[160][161]
A study using 50 mg/day progesterone by intramuscular injection in five men found that the medication produced azoospermia or severe oligozoospermia in all within 10 weeks, suppressed libido, erectile function, and ejaculate volume to minimal levels, produced slight gynecomastia in two of the men, moderately decreased testicular size, and impaired testicular morphology.[149][162][163][158][164][165][166] Upon discontinuation, sperm counts returned to normal in the men within 14 to 17 weeks.[149][162][158][164][166] In another study, 100 mg rectal suppositories of progesterone given five times per day for 9 days resulted in progesterone levels of 5.5 to 29 ng/mL and suppressed circulating testosterone and growth hormone levels by about 50% in men, but did not affect libido or erectile potency with this short duration of therapy.[149][167]
Progonadotropic effects
[edit]Progesterone can have progonadotropic effects under certain circumstances.[131]
Neurosteroid effects
[edit]Progesterone, through the actions of neurosteroid active metabolites such as allopregnanolone and pregnanolone, is a potent positive allosteric modulator of the GABAA receptor, the major signaling receptor of the inhibitory neurotransmitter γ-aminobutyric acid (GABA).[15] It can produce sedative, hypnotic, anxiolytic, euphoric, cognitive-, memory-, and motor-impairing, anticonvulsant, and even anesthetic effects with formation of sufficiently high concentrations of its neurosteroid metabolites and consequent GABAA receptor potentiation in the brain.[38][102][103][104] These actions and effects are characteristically similar to those of other GABAA receptor positive allosteric modulators like alcohol, barbiturates, and benzodiazepines.[104]
Similarly to other GABAA receptor positive allosteric modulators like alcohol, barbiturates, and benzodiazepines, tolerance has been found to develop with exposure to increased levels of allopregnanolone and related inhibitory neurosteroids.[168][169] This includes downregulation and desensitization of the GABAA receptor, reduced effects of allopregnanolone and other GABAA receptor activators (e.g., GABA and benzodiazepines), and rebound or withdrawal effects upon falls in allopregnanolone levels.[168][169] In addition, changes in allopregnanolone levels have been implicated in adverse neuropsychiatric effects associated with the menstrual cycle (e.g., dysphoria, depression, anxiety, irritability) and postpartum period (e.g., postpartum depression), as well as in catamenial epilepsy (seizures).[170][171] Low and high levels of allopregnanolone seem to have a neutral effect on mood, whereas moderate levels have a negative effect, which may underlie the symptoms of premenstrual syndrome and premenstrual dysphoric disorder that are observed in 30 to 40% of premenopausal women.[170][171][172] This U-shaped effect on mood appears to be a common property of GABAA receptor positive allosteric modulators.[170][171]
See also
[edit]References
[edit]- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- ^ Unfer, Vittorio; di Renzo, Gian; Gerli, Sandro; Casini, Maria (2006). "The Use of Progesterone in Clinical Practice: Evaluation of its Efficacy in Diverse Indications Using Different Routes of Administration". Current Drug Therapy. 1 (2): 211–219. doi:10.2174/157488506776930923. ISSN 1574-8855.
- ^ Whitaker, Amy; Gilliam, Melissa (2014). Contraception for Adolescent and Young Adult Women. Springer. p. 98. ISBN 9781461465799.
- ^ Chaudhuri (2007). Practice Of Fertility Control: A Comprehensive Manual (7Th ed.). Elsevier India. pp. 153–. ISBN 978-81-312-1150-2.
- ^ Attardi BJ, Burgenson J, Hild SA, Reel JR (March 2004). "In vitro antiprogestational/antiglucocorticoid activity and progestin and glucocorticoid receptor binding of the putative metabolites and synthetic derivatives of CDB-2914, CDB-4124, and mifepristone". J. Steroid Biochem. Mol. Biol. 88 (3): 277–88. doi:10.1016/j.jsbmb.2003.12.004. PMID 15120421. S2CID 23958876.
- ^ Soltysik K, Czekaj P (April 2013). "Membrane estrogen receptors - is it an alternative way of estrogen action?". J. Physiol. Pharmacol. 64 (2): 129–42. PMID 23756388.
- ^ Prossnitz ER, Barton M (May 2014). "Estrogen biology: New insights into GPER function and clinical opportunities". Mol. Cell. Endocrinol. 389 (1–2): 71–83. doi:10.1016/j.mce.2014.02.002. PMC 4040308. PMID 24530924.
- ^ a b c Rupprecht R, Reul JM, van Steensel B, Spengler D, Söder M, Berning B, Holsboer F, Damm K (October 1993). "Pharmacological and functional characterization of human mineralocorticoid and glucocorticoid receptor ligands". European Journal of Pharmacology. 247 (2): 145–54. doi:10.1016/0922-4106(93)90072-H. PMID 8282004.
- ^ a b c Elger W, Beier S, Pollow K, Garfield R, Shi SQ, Hillisch A (2003). "Conception and pharmacodynamic profile of drospirenone". Steroids. 68 (10–13): 891–905. doi:10.1016/j.steroids.2003.08.008. PMID 14667981. S2CID 41756726.
- ^ a b Attardi BJ, Zeleznik A, Simhan H, Chiao JP, Mattison DR, Caritis SN (2007). "Comparison of progesterone and glucocorticoid receptor binding and stimulation of gene expression by progesterone, 17-alpha hydroxyprogesterone caproate, and related progestins". Am. J. Obstet. Gynecol. 197 (6): 599.e1–7. doi:10.1016/j.ajog.2007.05.024. PMC 2278032. PMID 18060946.
- ^ a b Lei K, Chen L, Georgiou EX, Sooranna SR, Khanjani S, Brosens JJ, Bennett PR, Johnson MR (2012). "Progesterone acts via the nuclear glucocorticoid receptor to suppress IL-1β-induced COX-2 expression in human term myometrial cells". PLOS ONE. 7 (11): e50167. Bibcode:2012PLoSO...750167L. doi:10.1371/journal.pone.0050167. PMC 3509141. PMID 23209664.
- ^ a b Baulieu E, Schumacher M (2000). "Progesterone as a neuroactive neurosteroid, with special reference to the effect of progesterone on myelination". Steroids. 65 (10–11): 605–12. doi:10.1016/s0039-128x(00)00173-2. PMID 11108866. S2CID 14952168.
- ^ Maurice T, Urani A, Phan VL, Romieu P (November 2001). "The interaction between neuroactive steroids and the sigma1 receptor function: behavioral consequences and therapeutic opportunities". Brain Research. Brain Research Reviews. 37 (1–3): 116–32. doi:10.1016/s0165-0173(01)00112-6. PMID 11744080. S2CID 44931783.
- ^ Johannessen M, Fontanilla D, Mavlyutov T, Ruoho AE, Jackson MB (February 2011). "Antagonist action of progesterone at σ-receptors in the modulation of voltage-gated sodium channels". American Journal of Physiology. Cell Physiology. 300 (2): C328-37. doi:10.1152/ajpcell.00383.2010. PMC 3043630. PMID 21084640.
- ^ a b Paul SM, Purdy RH (March 1992). "Neuroactive steroids". FASEB Journal. 6 (6): 2311–22. doi:10.1096/fasebj.6.6.1347506. PMID 1347506. S2CID 221753076.
- ^ Knörr K, Beller FK, Lauritzen C (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 214–. ISBN 978-3-662-00942-0.
- ^ Knörr K, Knörr-Gärtner H, Beller FK, Lauritzen C (8 March 2013). Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. pp. 583–. ISBN 978-3-642-95583-9.
- ^ Labhart A (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 554–. ISBN 978-3-642-96158-8.
- ^ Horský J, Presl J (1981). "Hormonal Treatment of Disorders of the Menstrual Cycle". In Horsky J, Presl K (eds.). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 309–332. doi:10.1007/978-94-009-8195-9_11. ISBN 978-94-009-8195-9.
- ^ Ufer J (1969). The Principles and Practice of Hormone Therapy in Gynaecology and Obstetrics. de Gruyter. p. 49. ISBN 9783110006148.
17α-Hydroxyprogesterone caproate is a depot progestogen which is entirely free of side actions. The dose required to induce secretory changes in primed endometrium is about 250 mg. per menstrual cycle.
- ^ Pschyrembel W (1968). Praktische Gynäkologie: für Studierende und Ärzte. Walter de Gruyter. pp. 598, 601. ISBN 978-3-11-150424-7.
- ^ Ferin J (September 1972). "Effects, Duration of Action and Metabolism in Man". In Tausk M (ed.). Pharmacology of the Endocrine System and Related Drugs: Progesterone, Progestational Drugs and Antifertility Agents. Vol. II. Pergamon Press. pp. 13–24. ISBN 978-0080168128. OCLC 278011135.
- ^ Henzl MR, Edwards JA (10 November 1999). "Pharmacology of Progestins: 17α-Hydroxyprogesterone Derivatives and Progestins of the First and Second Generation". In Sitruk-Ware R, Mishell DR (eds.). Progestins and Antiprogestins in Clinical Practice. Taylor & Francis. pp. 101–132. ISBN 978-0-8247-8291-7.
- ^ Brotherton J (1976). Sex Hormone Pharmacology. Academic Press. p. 114. ISBN 978-0-12-137250-7.
- ^ Sang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–385. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.
- ^ Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- ^ Goebelsmann U (1986). "Pharmacokinetics of Contraceptive Steroids in Humans". In Gregoire AT, Blye RP (eds.). Contraceptive Steroids: Pharmacology and Safety. Springer Science & Business Media. pp. 67–111. doi:10.1007/978-1-4613-2241-2_4. ISBN 978-1-4613-2241-2.
- ^ Becker H, Düsterberg B, Klosterhalfen H (1980). "[Bioavailability of cyproterone acetate after oral and intramuscular application in men (author's transl)]" [Bioavailability of Cyproterone Acetate after Oral and Intramuscular Application in Men]. Urologia Internationalis. 35 (6): 381–385. doi:10.1159/000280353. PMID 6452729.
- ^ Moltz L, Haase F, Schwartz U, Hammerstein J (May 1983). "[Treatment of virilized women with intramuscular administration of cyproterone acetate]" [Efficacy of Intra muscularly Applied Cyproterone Acetate in Hyperandrogenism]. Geburtshilfe und Frauenheilkunde. 43 (5): 281–287. doi:10.1055/s-2008-1036893. PMID 6223851.
- ^ Wright JC, Burgess DJ (29 January 2012). Long Acting Injections and Implants. Springer Science & Business Media. pp. 114–. ISBN 978-1-4614-0554-2.
- ^ Chu YH, Li Q, Zhao ZF (April 1986). "Pharmacokinetics of megestrol acetate in women receiving IM injection of estradiol-megestrol long-acting injectable contraceptive". The Chinese Journal of Clinical Pharmacology.
The results showed that after injection the concentration of plasma MA increased rapidly. The meantime of peak plasma MA level was 3rd day, there was a linear relationship between log of plasma MA concentration and time (day) after administration in all subjects, elimination phase half-life t1/2β = 14.35 ± 9.1 days.
- ^ Runnebaum BC, Rabe T, Kiesel L (6 December 2012). Female Contraception: Update and Trends. Springer Science & Business Media. pp. 429–. ISBN 978-3-642-73790-9.
- ^ Artini PG, Genazzani AR, Petraglia F (11 December 2001). Advances in Gynecological Endocrinology. CRC Press. pp. 105–. ISBN 978-1-84214-071-0.
- ^ King TL, Brucker MC, Kriebs JM, Fahey JO (21 October 2013). Varney's Midwifery. Jones & Bartlett Publishers. pp. 495–. ISBN 978-1-284-02542-2.
- ^ a b c Elkik F, Mauvais-Jarvis P (January 1980). "Rôle de la progestérone et des progestatifs sur le métabolisme hydroélectrolytique" [The role of progesterone and progestins in hydroelectrolytic metabolism]. Nouv Presse Med (in French). 9 (1): 35–8. PMID 6986604.
- ^ a b Krattenmacher R (July 2000). "Drospirenone: pharmacology and pharmacokinetics of a unique progestogen". Contraception. 62 (1): 29–38. doi:10.1016/S0010-7824(00)00133-5. PMID 11024226.
- ^ a b c Simon JA (December 1995). "Micronized progesterone: vaginal and oral uses". Clinical Obstetrics and Gynecology. 38 (4): 902–14. doi:10.1097/00003081-199538040-00024. PMID 8616985.
- ^ a b c d Goletiani NV, Keith DR, Gorsky SJ (2007). "Progesterone: review of safety for clinical studies". Exp Clin Psychopharmacol. 15 (5): 427–44. doi:10.1037/1064-1297.15.5.427. PMID 17924777.
- ^ a b Oelkers W (2000). "Drospirenone--a new progestogen with antimineralocorticoid activity, resembling natural progesterone". Eur J Contracept Reprod Health Care. 5 (Suppl 3): 17–24. doi:10.1080/14730782.2000.12288986. PMID 11246598.
- ^ Quinkler M, Diederich S (November 2002). "Difference of in vivo and in vitro antimineralocorticoid potency of progesterone". Endocr Res. 28 (4): 465–70. doi:10.1081/erc-120016824. PMID 12530650. S2CID 168394.
- ^ Zerr-Fouineau M, Chataigneau M, Blot C, Schini-Kerth VB (January 2007). "Progestins overcome inhibition of platelet aggregation by endothelial cells by down-regulating endothelial NO synthase via glucocorticoid receptors". FASEB J. 21 (1): 265–73. doi:10.1096/fj.06-6840com. PMID 17116740. S2CID 22679638.
- ^ a b Fuhrmann U, Krattenmacher R, Slater EP, Fritzemeier KH (October 1996). "The novel progestin drospirenone and its natural counterpart progesterone: biochemical profile and antiandrogenic potential". Contraception. 54 (4): 243–51. doi:10.1016/s0010-7824(96)00195-3. PMID 8922878.
Drospirenone and progesterone exhibited low binding affinities to the rat GR as is documented by 1% and 11% RBA values compared to the reference dexamethasone, respectively. Similar results were reported elsewhere.8 In accordance with the low affinity to the GR, progesterone and drospirenone showed weak or no detectable agonistic activities, respectively, in the GR-dependent transactivation assay (Figure 2A and Figure 2B). Furthermore, both progestins were devoid of antiglucocorticoid activity in vitro. These data are in agreement with in vivo studies carried out with rats where drospirenone and progesterone showed neither glucocorticoid nor antiglucocorticoid activity.8
- ^ Wiegratz I, Kuhl H (August 2004). "Progestogen therapies: differences in clinical effects?". Trends Endocrinol. Metab. 15 (6): 277–85. doi:10.1016/j.tem.2004.06.006. PMID 15358281. S2CID 35891204.
- ^ Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- ^ a b c d e f Yeh YT, Chang CW, Wei RJ, Wang SN (2013). "Progesterone and related compounds in hepatocellular carcinoma: basic and clinical aspects". Biomed Res Int. 2013: 290575. doi:10.1155/2013/290575. PMC 3581253. PMID 23484104.
- ^ a b c Ottosson UB (1984). "Oral progesterone and estrogen/progestogen therapy. Effects of natural and synthetic hormones on subfractions of HDL cholesterol and liver proteins". Acta Obstet Gynecol Scand Suppl. 127: 1–37. doi:10.3109/00016348409157016. PMID 6596830. S2CID 26138417.
Natural progesterone is devoid of any androgenic activity that might compromise lipoprotein metabolism or induce teratogenicity.
- ^ Zutshi (2005). Hormones in Obstetrics and Gynaecology. Jaypee Brothers, Medical Publishers. pp. 74–75. ISBN 978-81-8061-427-9.
It has been observed that micronized progesterone has no suppressive effects on high-density lipoprotein-cholesterol (HDL-C). Jensen et al have proved that oral micronized progesterone has no adverse effect on serum lipids. These preparations have the same antiestrogenic and antimineralocorticoid effect but no androgenic action. It does not affect aldosterone synthesis, blood pressure, carbohydrate metabolism or mood changes. No side effects have been reported as far as lipid profile, coagulation factors and blood pressure are concerned.
[permanent dead link] - ^ Levy T, Yairi Y, Bar-Hava I, Shalev J, Orvieto R, Ben-Rafael Z (2000). "Pharmacokinetics of the progesterone-containing vaginal tablet and its use in assisted reproduction" (PDF). Steroids. 65 (10–11): 645–9. doi:10.1016/s0039-128x(00)00121-5. PMID 11108871. S2CID 9627000.
Natural progesterone is devoid of any androgenic activity and is thus extensively used in assisted reproduction, sometimes for long periods of time.
- ^ Samsioe, Göran; Dören, Martina; Lobo, Rogerio A (2006). "Hormone replacement therapy – the agents". Women's Health Medicine. 3 (5): 213–216. doi:10.1053/S1744-1870(06)70207-4. ISSN 1744-1870.
Progestogens differ in their relative metabolic and androgenic effects; for example MPA is minimally androgenic, but does counteract the rise in HDL-cholesterol caused by oestrogen therapy. In contrast, oral micronized progesterone does not mitigate against increased HDL-cholesterol levels.
- ^ a b Woods KS, Reyna R, Azziz R (2002). "Effect of oral micronized progesterone on androgen levels in women with polycystic ovary syndrome". Fertil. Steril. 77 (6): 1125–7. doi:10.1016/s0015-0282(02)03119-9. PMID 12057716.
The mean values of TT, FT, SHBG, DHEAS, A4, and 17-OHP did not change with OMP administration. However, a higher 17-OHP level was observable at the completion of OMP administration (week 2).
- ^ Sitruk-Ware R (2002). "Progestogens in hormonal replacement therapy: new molecules, risks, and benefits". Menopause. 9 (1): 6–15. doi:10.1097/00042192-200201000-00003. PMID 11791081. S2CID 12136231.
- ^ a b Sumino, Hiroyuki; Ichikawa, Shuichi; Kasama, Shu; Takahashi, Takashi; Kumakura, Hisao; Takayama, Yoshiaki; Minami, Kazutomo; Kanda, Tsugiyasu; Kurabayashi, Masahiko; Murakami, Masami (2011). "Hormone Therapy and Blood Pressure in Postmenopausal Women". Journal of Experimental & Clinical Medicine. 3 (3): 112–115. doi:10.1016/j.jecm.2011.04.005. ISSN 1878-3317.
Natural progesterone, such as micronized progesterone, has no androgenic properties, whereas some synthetic progestins, such as MPA and norethisterone acetate, possess androgenic side effects, which raise the concern of potentially harmful effects on blood pressure.
- ^ Walch KT, Huber JC (April 2008). "Progesterone for recurrent miscarriage: truth and deceptions". Best Pract Res Clin Obstet Gynaecol. 22 (2): 375–89. doi:10.1016/j.bpobgyn.2007.08.009. PMID 17964858.
- ^ Rode L, Langhoff-Roos J, Andersson C, Dinesen J, Hammerum MS, Mohapeloa H, Tabor A (2009). "Systematic review of progesterone for the prevention of preterm birth in singleton pregnancies". Acta Obstet Gynecol Scand. 88 (11): 1180–9. doi:10.3109/00016340903280982. PMID 19900136. S2CID 556588.
- ^ a b c Häggström, Mikael; Richfield, David (2014). "Diagram of the pathways of human steroidogenesis". WikiJournal of Medicine. 1 (1). doi:10.15347/wjm/2014.005. ISSN 2002-4436.
- ^ a b Samuel B. Frank (1971). Acne Vulgaris. Thomas. p. 131. ISBN 9780398006020.
The chemical structure of progesterone and testosterone are remarkably similar; they differ only in the side chain at the 17-carbon position. The possibility that progesterone can be transformed to testosterone has been considered good by many. If true, it could then be a source of androgens in women. [...] Laboratory evidence exists that progesterone can be converted to testosterone in vitro by human and animal ovarian and testicular tissue.44-47 Although the role of progesterone in acne and its effect on sebaceous gland activity is not fully established, the possibility that endogenous progesterone is a precursor of testosterone or of another androgenic substance invites further exploration.48,49
- ^ Vermorken, A. J. M.; Houben, J. J. G. (2016). "Topical Androgen Treatment for ACNE a Review". Drug Intelligence & Clinical Pharmacy. 12 (3): 151–157. doi:10.1177/106002807801200302. ISSN 0012-6578. S2CID 74413605.
The only concern Voigt and Hsia expressed about the use of progesterone as an anti-androgen was the possibility that the small amount of hormone which reached the circulation could be converted into testosterone by the sexual organs, mainly the testes.
- ^ Shufeng Zhou (6 April 2016). Cytochrome P450 2D6: Structure, Function, Regulation and Polymorphism. CRC Press. pp. 52–. ISBN 978-1-4665-9788-4.
- ^ a b Whitehead MI, Townsend PT, Gill DK, Collins WP, Campbell S (1980). "Absorption and metabolism of oral progesterone". Br Med J. 280 (6217): 825–7. doi:10.1136/bmj.280.6217.825. PMC 1600943. PMID 7370683.
Plasma concentrations of oestradiol were unchanged by giving progesterone.
- ^ a b Dewis P, Newman M, Anderson DC (October 1984). "The effect of endogenous progesterone on serum levels of 5α-reduced androgens in hirsute women". Clin. Endocrinol. (Oxf). 21 (4): 383–92. doi:10.1111/j.1365-2265.1984.tb03225.x. PMID 6542470. S2CID 72895292.
These studies suggest that [...] a rise in serum progesterone has only a minimal effect on circulating levels of the active 5α‐reduced androgen metabolites. [...] Progesterone has been shown to be a potent in vitro inhibitor of cutaneous 5α-reductase (Mauvais-Jarvis et al., 1974). However we found only a small reduction in serum DHT levels in the late luteal phase in ovulatory women and no change in serum 3α-diol. Hence the rise in serum progesterone in ovulatory women has only a minimal effect on the circulating levels of the major active 5α-reduced androgens in vivo.
- ^ Rižner TL, Brožič P, Doucette C, Turek-Etienne T, Müller-Vieira U, Sonneveld E, van der Burg B, Böcker C, Husen B (May 2011). "Selectivity and potency of the retroprogesterone dydrogesterone in vitro". Steroids. 76 (6): 607–15. doi:10.1016/j.steroids.2011.02.043. PMID 21376746. S2CID 31609405.
- ^ Swerdloff RS, Dudley RE, Page ST, Wang C, Salameh WA (2017). "Dihydrotestosterone: Biochemistry, Physiology, and Clinical Implications of Elevated Blood Levels". Endocr. Rev. 38 (3): 220–254. doi:10.1210/er.2016-1067. PMC 6459338. PMID 28472278.
- ^ a b Marks LS (2004). "5α-reductase: history and clinical importance". Rev Urol. 6 (Suppl 9): S11–21. PMC 1472916. PMID 16985920.
- ^ Golub MS, Kaufman FL, Campbell MA, Li LH, Donald JM (October 2006). ""Natural" progesterone: information on fetal effects". Birth Defects Research Part B: Developmental and Reproductive Toxicology. 77 (5): 455–70. doi:10.1002/bdrb.20089. PMID 17066418.
Progesterone has been shown to inhibit 5α-reductase, another important enzyme in steroid hormone metabolism, (Dean and Winter, 1984; Beckmann et al., 1993; Cassidenti et al., 1991; Kadohama et al., 1983; Mauvais-Jarvis et al., 1974; Dube et al., 1975). However, this is a weak effect that has only been demonstrated at supra-physiological concentrations and in vitro conditions.
- ^ Kincl, Fred A. (1990). "Control of Reproductive Function in the Adult". Hormone Toxicity in the Newborn. Monographs on Endocrinology. Vol. 31. pp. 5–120. doi:10.1007/978-3-642-83794-4_2. ISBN 978-3-642-83796-8. ISSN 0077-1015. PMID 1965221.
Progesterone (and other progestational agents) inhibit testosterone from expressing its activity at the target sites (Kincl, 1971a). Mice and rats are the test animals of choice (Dorfman, 1963a,b). Inhibition of 5α-reductase activity of binding to cytosol and nuclear receptors has been shown to be the steps at which antiandrogens express their activity (Neumann and Steinbeck, 1974). Relatively high amounts are needed to achieve a significant effect (Table 2.16).
- ^ Kålund-Jensen H, Myrén CJ (December 1984). "Vaginal absorption of oestradiol and progesterone". Maturitas. 6 (4): 359–67. doi:10.1016/0378-5122(84)90009-4. PMID 6543461.
- ^ Picut CA, Ziejewski MK, Stanislaus D (February 2018). "Comparative Aspects of Pre- and Postnatal Development of the Male Reproductive System". Birth Defects Res. 110 (3): 190–227. doi:10.1002/bdr2.1133. PMID 29063715. S2CID 3967093.
- ^ a b Sitruk-Ware R (August 2018). "Non-clinical studies of progesterone". Climacteric. 21 (4): 315–320. doi:10.1080/13697137.2018.1463982. PMC 6281289. PMID 29790373.
- ^ Fred A. Kincl (6 December 2012). Hormone Toxicity in the Newborn. Springer Science & Business Media. p. 60. ISBN 978-3-642-83794-4.
- ^ Kawashima K, Nakaura S, Nagao S, Tanaka S, Kuwamura T (February 1977). "Virilizing activities of various steroids in female rat fetuses". Endocrinol. Jpn. 24 (1): 77–81. doi:10.1507/endocrj1954.24.77. PMID 558879.
- ^ a b c d e f g Aufrère MB, Benson H (June 1976). "Progesterone: an overview and recent advances". J Pharm Sci. 65 (6): 783–800. doi:10.1002/jps.2600650602. PMID 945344.
Early studies on its use as an oral contraceptive showed that, at 300 mg/day (5th to 25th day of the menstrual cycle), progesterone was effective in preventing ovulation through four cycles (263). The related effect of larger doses of progesterone on gonadotropin excretion also has been investigated. Rothchild (264) found that continuous or intermittent intravenously administered progesterone (100-400 mg/day) for 10 days depressed the total amount of gonadotropin excreted into the urine. However, Paulsen et al. (265) found that oral progesterone at 1000 mg/day for 87 days did not have a significant effect on urinary gonadotropin excretion. The efficacy of progesterone as an oral contraceptive was never fully tested, because synthetic progestational agents, which were orally effective, were available.
- ^ Tony M. Plant; Anthony J. Zeleznik (15 November 2014). Knobil and Neill's Physiology of Reproduction. Academic Press. pp. 2289, 2386. ISBN 978-0-12-397769-4.
- ^ Hormones, Brain and Behavior, Five-Volume Set. Elsevier. 18 June 2002. pp. 54–. ISBN 978-0-08-053415-2.
- ^ Heidrich A, Schleyer M, Spingler H, Albert P, Knoche M, Fritze J, Lanczik M (February 1994). "Postpartum blues: relationship between not-protein bound steroid hormones in plasma and postpartum mood changes". J Affect Disord. 30 (2): 93–8. doi:10.1016/0165-0327(94)90036-1. PMID 8201129.
- ^ Baek, K.; Rosenwaks, Z.; Poppas, D.P.; Palermo, G.D. (2006). "P-657". Fertility and Sterility. 86 (3): S377. doi:10.1016/j.fertnstert.2006.07.1033. ISSN 0015-0282.
- ^ Pharmacology of the Skin I: Pharmacology of Skin Systems Autocoids in Normal and Inflamed Skin. Springer Science & Business Media. 6 December 2012. pp. 249–250. ISBN 978-3-642-73797-8.
- ^ a b Pharmacology of the Skin II: Methods, Absorption, Metabolism and Toxicity, Drugs and Diseases. Springer Science & Business Media. 6 December 2012. pp. 253, 485–. ISBN 978-3-642-74054-1.
- ^ a b Walter P. Unger (1 February 1995). "Androgenetic alopecia and its treatment. A historical overview". Hair Transplantation, Third Edition. Taylor & Francis. pp. 1–33. ISBN 978-0-8247-9363-0.
- ^ a b Sawaya, Marty E.; Shapiro, Jerry (2000). "Androgenetic alopecia". Dermatologic Clinics. 18 (1): 47–61. doi:10.1016/S0733-8635(05)70146-7. ISSN 0733-8635. PMID 10626111.
- ^ Parthasarathy, Saudhamini; Chin, Andrea; Malloy, Virginia; Matias, Jonathan (1988). "In Vitro Androgen Receptor Binding Affinity and in Vivo Inhibitory Activity of 5?-Pregnane-3, 20-Dione". Annals of the New York Academy of Sciences. 529 (1 Fourth Colloq): 239–241. Bibcode:1988NYASA.529..239P. doi:10.1111/j.1749-6632.1988.tb51470.x. ISSN 0077-8923. S2CID 86039350.
- ^ Price, Vera H. (1988). "Androgenetic alopecia and hair growth promotion state of the art: Present and future". Clinics in Dermatology. 6 (4): 218–227. doi:10.1016/0738-081X(88)90090-9. ISSN 0738-081X. PMID 3063373.
- ^ Sawaya ME, Hordinsky MK (January 1993). "The antiandrogens. When and how they should be used". Dermatol Clin. 11 (1): 65–72. doi:10.1016/S0733-8635(18)30283-3. PMID 8435919.
- ^ a b c Neubauer H, Ma Q, Zhou J, Yu Q, Ruan X, Seeger H, Fehm T, Mueck AO (October 2013). "Possible role of PGRMC1 in breast cancer development". Climacteric. 16 (5): 509–13. doi:10.3109/13697137.2013.800038. PMID 23758160. S2CID 29808177.
- ^ Ruan X, Neubauer H, Yang Y, Schneck H, Schultz S, Fehm T, Cahill MA, Seeger H, Mueck AO (October 2012). "Progestogens and membrane-initiated effects on the proliferation of human breast cancer cells". Climacteric. 15 (5): 467–72. doi:10.3109/13697137.2011.648232. PMID 22335423. S2CID 11302554.
- ^ a b c d Trabert B, Sherman ME, Kannan N, Stanczyk FZ (September 2019). "Progesterone and breast cancer". Endocr. Rev. 41 (2): 320–344. doi:10.1210/endrev/bnz001. PMC 7156851. PMID 31512725.
- ^ a b c P. J. Bentley (1980). Endocrine Pharmacology: Physiological Basis and Therapeutic Applications. CUP Archive. pp. 264, 274. ISBN 978-0-521-22673-8.
- ^ a b Mark A. Sperling (10 April 2014). Pediatric Endocrinology E-Book. Elsevier Health Sciences. pp. 630–. ISBN 978-1-4557-5973-6.
- ^ Sue Macdonald; Gail Johnson (3 June 2017). Mayes' Midwifery E-Book. Elsevier Health Sciences. pp. 391–. ISBN 978-0-7020-6336-7.
- ^ Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 889, 940. ISBN 978-0-7817-1750-2.
- ^ Lee-Ellen C. Copstead-Kirkhorn; Jacquelyn L. Banasik (25 June 2014). Pathophysiology - E-Book. Elsevier Health Sciences. pp. 660–. ISBN 978-0-323-29317-4.
Throughout the reproductive years, some women note swelling of the breast around the latter part of each menstrual cycle before the onset of menstruation. The water retention and subsequent swelling of breast tissue during this phase of the menstrual cycle are thought to be due to high levels of circulating progesterone stimulating the secretory cells of the breast.12
- ^ Farage MA, Neill S, MacLean AB (2009). "Physiological changes associated with the menstrual cycle: a review". Obstet Gynecol Surv. 64 (1): 58–72. doi:10.1097/OGX.0b013e3181932a37. PMID 19099613. S2CID 22293838.
- ^ Raine-Fenning NJ, Brincat MP, Muscat-Baron Y (2003). "Skin aging and menopause : implications for treatment". Am J Clin Dermatol. 4 (6): 371–8. doi:10.2165/00128071-200304060-00001. PMID 12762829. S2CID 20392538.
- ^ Holzer G, Riegler E, Hönigsmann H, Farokhnia S, Schmidt JB, Schmidt B (2005). "Effects and side-effects of 2% progesterone cream on the skin of peri- and postmenopausal women: results from a double-blind, vehicle-controlled, randomized study". Br. J. Dermatol. 153 (3): 626–34. doi:10.1111/j.1365-2133.2005.06685.x. PMID 16120154. S2CID 6077829.
- ^ a b Leon Speroff; Marc A. Fritz (2005). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 211–. ISBN 978-0-7817-4795-0.
When administered before the estrogen stimulus, or in high doses (achieving a blood level greater than 2 ng/mL), progesterone blocks the midcycle LH surge.
- ^ Charles R. B. Beckmann; William Herbert; Douglas Laube; Frank Ling, Roger Smith (21 January 2013). Obstetrics and Gynecology. Lippincott Williams & Wilkins. pp. 342–. ISBN 978-1-4698-2604-2.
- ^ Quigley MM (August 1986). "Drugs in the treatment of female infertility. Recent advances". Drugs. 32 (2): 169–77. doi:10.2165/00003495-198632020-00004. PMID 3527660. S2CID 46972235.
In the presence of circulating levels of approximately 4 μg/L or greater of progesterone, most women experience a 0.5° to 1°F rise in basal body temperature.
- ^ Shanafelt TD, Barton DL, Adjei AA, Loprinzi CL (2002). "Pathophysiology and treatment of hot flashes". Mayo Clin. Proc. 77 (11): 1207–18. doi:10.4065/77.11.1207. PMID 12440557.
- ^ Sassarini J, Lumsden MA (2010). "Hot flushes: are there effective alternatives to estrogen?". Menopause Int. 16 (2): 81–8. doi:10.1258/mi.2010.010007. PMID 20729500. S2CID 37505358.
- ^ Bayliss DA, Millhorn DE (1992). "Central neural mechanisms of progesterone action: application to the respiratory system". J. Appl. Physiol. 73 (2): 393–404. doi:10.1152/jappl.1992.73.2.393. PMID 1399957.
- ^ Ghada Bourjeily; Karen Rosene-Montella (21 April 2009). Pulmonary Problems in Pregnancy. Springer Science & Business Media. pp. 21–. ISBN 978-1-59745-445-2.
- ^ a b Gompel A, Plu-Bureau G (August 2018). "Progesterone, progestins and the breast in menopause treatment". Climacteric. 21 (4): 326–332. doi:10.1080/13697137.2018.1476483. PMID 29852797. S2CID 46922084.
- ^ a b Wang-Cheng R, Neuner JM, Barnabei VM (2007). Menopause. ACP Press. p. 97. ISBN 978-1-930513-83-9.
- ^ a b Bergemann N, Ariecher-Rössler A (27 December 2005). Estrogen Effects in Psychiatric Disorders. Springer Science & Business Media. p. 179. ISBN 978-3-211-27063-9.
- ^ a b c Bäckström T, Bixo M, Johansson M, Nyberg S, Ossewaarde L, Ragagnin G, Savic I, Strömberg J, Timby E, van Broekhoven F, van Wingen G (2014). "Allopregnanolone and mood disorders". Prog. Neurobiol. 113: 88–94. doi:10.1016/j.pneurobio.2013.07.005. PMID 23978486. S2CID 207407084.
- ^ Eric J. Bieber; Joseph S. Sanfilippo; Ira R. Horowitz; Mahmood I. Shafi (23 April 2015). Clinical Gynecology. Cambridge University Press. pp. 972–. ISBN 978-1-107-04039-7.
- ^ Susan Tucker Blackburn (2007). Maternal, Fetal, & Neonatal Physiology: A Clinical Perspective. Elsevier Health Sciences. pp. 44–. ISBN 978-1-4160-2944-1.
- ^ J. Larry Jameson; Leslie J. De Groot (25 February 2015). Endocrinology: Adult and Pediatric E-Book. Elsevier Health Sciences. pp. 1–. ISBN 978-0-323-32195-2.
- ^ Younis JS, Simon A, Laufer N (December 1996). "Endometrial preparation: lessons from oocyte donation". Fertil Steril. 66 (6): 873–84. doi:10.1016/s0015-0282(16)58677-4. PMID 8941049.
It seems that [progesterone] levels of approximately 10 ng/mL are sufficient to sustain morphological and functional endometrial development. [...] Oral micronized P (100 mg three times) was investigated in ovarian failure patients in 12 cycles after adequate E2 priming (84). The serum P levels achieved were subphysiologic and incapable of inducing a normal secretory response in the endometrium.
- ^ Howard Carp (13 June 2007). Recurrent Pregnancy Loss: Causes, Controversies and Treatment. CRC Press. pp. 79–. ISBN 978-0-415-42130-0.
- ^ a b Gautam N Allahbadia; Rita Basuray Das; Goral Gandhi; Rubina Merchant (17 July 2017). The Art & Science of Assisted Reproductive Techniques (ART). JP Medical Ltd. pp. 145–. ISBN 978-93-86322-82-1.
- ^ Lars Philip Bengtsson; M. Tausk (30 January 1971). Pharmacology of the endocrine system and related drugs: progesterone, progestational drugs and antifertility agents. Pergamon Press. p. 449. ISBN 9780080157450.
- ^ a b de Ziegler D, Sator M, Binelli D, Leuratti C, Cometti B, Bourgain C, Fu YS, Garhöfer G (September 2013). "A randomized trial comparing the endometrial effects of daily subcutaneous administration of 25 mg and 50 mg progesterone in aqueous preparation". Fertil. Steril. 100 (3): 860–6. doi:10.1016/j.fertnstert.2013.05.029. PMID 23806850.
- ^ a b c d de Ziegler D, Fanchin R (2000). "Progesterone and progestins: applications in gynecology". Steroids. 65 (10–11): 671–9. doi:10.1016/s0039-128x(00)00123-9. PMID 11108875. S2CID 5867301.
- ^ Stanczyk FZ (2014). "Treatment of postmenopausal women with topical progesterone creams and gels: are they effective?". Climacteric. 17 (Suppl 2): 8–11. doi:10.3109/13697137.2014.944496. PMID 25196424. S2CID 20019151.
- ^ Stanczyk FZ, Paulson RJ, Roy S (2005). "Percutaneous administration of progesterone: blood levels and endometrial protection". Menopause. 12 (2): 232–7. doi:10.1097/00042192-200512020-00019. PMID 15772572. S2CID 10982395.
- ^ Gautam N. Allahbadia; Yoshiharu Morimoto (15 September 2015). Ovarian Stimulation Protocols. Springer. pp. 137–. ISBN 978-81-322-1121-1.
- ^ Devroey P, Palermo G, Bourgain C, Van Waesberghe L, Smitz J, Van Steirteghem AC (1989). "Progesterone administration in patients with absent ovaries". Int. J. Fertil. 34 (3): 188–93. PMID 2567713.
- ^ Stute P, Neulen J, Wildt L (August 2016). "The impact of micronized progesterone on the endometrium: a systematic review". Climacteric. 19 (4): 316–28. doi:10.1080/13697137.2016.1187123. PMID 27277331.
- ^ Davey DA (October 2018). "Menopausal hormone therapy: a better and safer future". Climacteric. 21 (5): 454–461. doi:10.1080/13697137.2018.1439915. PMID 29526116. S2CID 3850275.
- ^ a b Pasqualini JR (2007). "Progestins and breast cancer". Gynecol. Endocrinol. 23 (Suppl 1): 32–41. doi:10.1080/09513590701585003. PMID 17943537. S2CID 46634314.
- ^ a b Pasqualini JR (2009). "Breast cancer and steroid metabolizing enzymes: the role of progestogens". Maturitas. 65 (Suppl 1): S17–21. doi:10.1016/j.maturitas.2009.11.006. PMID 19962254.
- ^ Kopernik G, Shoham Z (June 2004). "Tools for making correct decisions regarding hormone therapy. Part II. Organ response and clinical applications". Fertil. Steril. 81 (6): 1458–77. doi:10.1016/j.fertnstert.2003.09.080. PMID 15193461.
- ^ Gorins A, Denis C (1995). "[Effects of progesterone and progestational hormones on the mammary gland]". Arch Anat Cytol Pathol (in French). 43 (1–2): 28–35. PMID 7794024.
- ^ de Lignières B (September 2002). "Effects of progestogens on the postmenopausal breast". Climacteric. 5 (3): 229–35. doi:10.1080/713605271. PMID 12419080.
- ^ Barrat J, de Lignières B, Marpeau L, Larue L, Fournier S, Nahoul K, Linares G, Giorgi H, Contesso G (1990). "Effet in vivo de l'administration locale de progestérone sur l'activité mitotique des galactophores humains: résultat d'une étude pilote" [The in vivo effect of the local administration of progesterone on the mitotic activity of human ductal breast tissue. Results of a pilot study]. J Gynecol Obstet Biol Reprod (Paris) (in French). 19 (3): 269–74. PMID 2345268.
- ^ Chang KJ, Lee TT, Linares-Cruz G, Fournier S, de Ligniéres B (April 1995). "Influences of percutaneous administration of estradiol and progesterone on human breast epithelial cell cycle in vivo". Fertil. Steril. 63 (4): 785–91. doi:10.1016/S0015-0282(16)57482-2. PMID 7890063.
- ^ a b Spicer DV, Ursin G, Pike MC (May 1996). "Progesterone concentrations--physiologic or pharmacologic?". Fertil. Steril. 65 (5): 1077–8. doi:10.1016/s0015-0282(16)58295-8. PMID 8612843.
- ^ J. M. Foidart; C. Colin; X. Denoo; J. D. Desreux; S. Fournier; B. de Lignieres (1996). "Influence of percutaneous administration of estradiol and progesterone on the proliferation of human breast epithelial cells". In F. Calvo; M. Crepin; H. Magdelenat (eds.). Breast Cancer Advances in Biology and Therapeutics. John Libbey Eurotext. pp. 329–334. ISBN 9782742001385.
- ^ Foidart JM, Colin C, Denoo X, Desreux J, Béliard A, Fournier S, de Lignières B (May 1998). "Estradiol and progesterone regulate the proliferation of human breast epithelial cells". Fertil. Steril. 69 (5): 963–9. doi:10.1016/s0015-0282(98)00042-9. PMID 9591509.
- ^ a b c d de Lignières B, Silberstein S (April 2000). "Pharmacodynamics of oestrogens and progestogens". Cephalalgia: An International Journal of Headache. 20 (3): 200–7. doi:10.1046/j.1468-2982.2000.00042.x. PMID 10997774. S2CID 40392817.
- ^ a b Shaw RW (November 1978). "Neuroendocrinology of the menstrual cycle in humans". Clin Endocrinol Metab. 7 (3): 531–59. doi:10.1016/S0300-595X(78)80008-5. PMID 365398.
- ^ a b Endrikat J, Gerlinger C, Richard S, Rosenbaum P, Düsterberg B (December 2011). "Ovulation inhibition doses of progestins: a systematic review of the available literature and of marketed preparations worldwide". Contraception. 84 (6): 549–57. doi:10.1016/j.contraception.2011.04.009. PMID 22078182.
Table 1 Publications on ovulation inhibition doses of progestins: Progestin: Progesterone. Reference: Pincus (1956). Method: Urinary pregnanediol. Daily dose (mg): 300.000. Total number of cycles in all subjects: 61. Total number of ovulation in all subjects: 30. % of ovulation in all subjects: 49.
- ^ a b Stone, Abraham; Kupperman, Herbert S. (1955). "The Effects of Progesterone on Ovulation: A Preliminary Report". The Fifth International Conference on Planned Parenthood: Theme, Overpopulation and Family Planning: Report of the Proceedings, 24-29 October, 1955, Tokyo, Japan. Vol. 33. International Planned Parenthood Federation. p. 202. doi:10.1016/0093-691x(90)90626-5.
The results of testing the effects of progesterone on ovulation in 13 patients at the Margaret Sanger Research Bureau are presented. The patients had normal menstrual cycles and showed clear evidence of ovulation. Each patient was given 1000 [mg] of [oral] progesterone daily during the midperiod for 10 or 12 days during 16 cycles. Ovulation was inhibited in 6 cycles. No disturbance in menstrual rhythm was observed. 3 of 12 patients with longstanding infertility histories became pregnant within 2-4 months after the cessation of progesterone therapy.
- ^ a b S. Beier; B. Düsterberg; M. F. El Etreby; W. Elger; F. Neumann; Y. Nishino (1983). "Toxicology of Hormonal Fertility Regulating Agents". In Giuseppe Benagiano; Egon Diczfalusy (eds.). Endocrine Mechanisms in Fertility Regulation. Raven Press. pp. 261–346. ISBN 978-0-89004-464-3.
- ^ Pincus G (1956). "Some effects of progesterone and related compounds upon reproduction and early development in mammals". Acta Endocrinol Suppl (Copenh). 23 (Suppl 28): 18–36. doi:10.1530/acta.0.023S018. PMID 13394044.
- ^ Pincus G (December 1958). "The hormonal control of ovulation and early development". Postgrad Med. 24 (6): 654–60. doi:10.1080/00325481.1958.11692305. PMID 13614060.
Table 1: Effects of oral progesterone on three indexes of ovulation: Medication: Progesterone. Number: 69. Mean cycle length: 25.5 ± 0.59. Per cent positive for ovulation by: Basal temperature: 27. Endometrial biopsy: 18. Vaginal smear: 6. [...] we settled on 300 mg. per day [oral progersterone] as a significantly effective [ovulation inhibition] dosage, and this was administered from the fifth day through the twenty-fourth day of the menstrual cycle. [...] We observed each of 33 volunteer subjects during a control, nontreatment cycle and for one to three successive cycles of medication immediately following the control cycle. As indexes of the occurrence of ovulation, daily basal temperatures and vaginal smears were taken, and at the nineteenth to twenty-second day of the cycle an endometrial biopsy. [...] Although we thus demonstrated the ovulation-inhibiting activity of progesterone in normally ovulating women, oral progesterone medication had two disadvantages: ( l) the large daily dosage ( 300 mg.) which presumably would have to be even larger if one sought 100 per cent inhibition1 [...]
- ^ a b Pincus, Gregory (1959). Progestational Agents and the Control of Fertility. Vitamins & Hormones. Vol. 17. pp. 307–324. doi:10.1016/S0083-6729(08)60274-5. ISBN 9780127098173. ISSN 0083-6729.
Ishikawa et al. (1957) employing the same regime of progesterone administration also observed suppression of ovulation in a proportion of the cases taken to laparotomy. Although sexual intercourse was practised freely by the subjects of our experiments and those of Ishikawa el al., no pregnancies OCcurred. Since ovulation presumably took place in a proportion of cycles, the lack of any pregnancies may be due to chance, but Ishikawa et al. (1957) have presented data indicating that in women receiving oral progesterone the cervical mucus becomes impenetrable to sperm.
- ^ Rock J, Garcia CR, Pincus G (1957). "Synthetic progestins in the normal human menstrual cycle". Recent Prog. Horm. Res. 13: 323–39, discussion 339–46. PMID 13477811.
- ^ Tyler ET, Olson HJ (April 1959). "Fertility promoting and inhibiting effects of new steroid hormonal substances". J Am Med Assoc. 169 (16): 1843–54. doi:10.1001/jama.1959.03000330015003. PMID 13640942.
- ^ Haller, J. (1968). "Die antikonzeptionelle Therapie". Die Gestagene. pp. 1125–1178. doi:10.1007/978-3-642-99941-3_8. ISBN 978-3-642-99942-0.
- ^ Neumann FW, Elger Y, Nishino Y, Steinbeck H (1977). "Probleme der Dosisfindung: Sexualhormone" [Problems of Dose-Finding: Sex Hormones]. Arzneimittel-Forschung [Drug Research]. 27: 296–318. ISSN 0004-4172.
- ^ Neumann F (1978). "The physiological action of progesterone and the pharmacological effects of progestogens--a short review". Postgrad Med J. 54 (Suppl 2): 11–24. PMID 368741.
- ^ Neumann, F (1987). "Pharmacology and Clinical Uses of Cyproterone Acetate". In Furr, BJA; Wakeling, AE (eds.). Pharmacology and Clinical Uses of Inhibitors of Hormone Secretion and Action. London: Baillière Tindall. pp. 132–159. ISBN 9780702011368. OCLC 925173670. OL 20778637M.
- ^ Victor A, Jackanicz TM, Johansson ED (December 1978). "Vaginal progesterone for contraception". Fertil. Steril. 30 (6): 631–5. doi:10.1016/S0015-0282(16)43688-5. PMID 729823.
- ^ Croxatto HB, Díaz S (1987). "The place of progesterone in human contraception". J. Steroid Biochem. 27 (4–6): 991–4. doi:10.1016/0022-4731(87)90179-8. PMID 3320572.
- ^ Bäckström T, von Schoultz B, Toivonen J (1979). "Plasma progesterone concentrations after administration via intravaginal rings". Acta Obstet Gynecol Scand. 58 (2): 211–2. doi:10.3109/00016347909154585. PMID 452876. S2CID 34956863.
- ^ Shaaban MM (1991). "Contraception with progestogens and progesterone during lactation". J. Steroid Biochem. Mol. Biol. 40 (4–6): 705–10. doi:10.1016/0960-0760(91)90294-F. PMID 1835650. S2CID 25152238.
- ^ Wadsworth PF, Heywood R, Allen DG, Hossack DJ, Sortwell RJ, Walton RM (October 1979). "Treatment of rhesus monkeys (Macaca mulatta) with intravaginal rings impregnated with either progesterone or norethisterone". Contraception. 20 (4): 339–51. doi:10.1016/s0010-7824(79)80044-x. PMID 116799.
- ^ a b c d Janet Brotherton (1976). Sex Hormone Pharmacology. Academic Press. pp. 220, 341–342. ISBN 978-0-12-137250-7.
- ^ Hans-Dieter Taubert; Herbert Kuhl (1981). Kontrazeption mit Hormonen [Contraception with Hormones] (1 ed.). Stuttgart/New York: Goerg Thieme Verlag. p. 86. ISBN 9783136088012. OCLC 612130880.
Daily intramuscular injection of 5 to 10 mg of progesterone from days 7 to 23 suppresses LH and FSH and prevents ovulation (569). Progestogens also lead to a reduction in gonadotropin levels.
- ^ Netter A, Gorins A, Thomas K, Cohen M, Joubinaux J (1973). "Blocage du pic d'ovulation de LH et de FSH par la progesterone à faibles doses chez la femme" [Blockade of LH and FSH peaks by low doses of exogenous progesterone in the human female]. Ann. Endocrinol. (Paris) (in French). 34 (4): 430–5. ISSN 0003-4266. PMID 4779738.
- ^ Lobo, Rogerio A.; Stanczyk, Frank Z. (1994). "New knowledge in the physiology of hormonal contraceptives". American Journal of Obstetrics and Gynecology. 170 (5): 1499–1507. doi:10.1016/S0002-9378(12)91807-4. ISSN 0002-9378. PMID 8178898.
- ^ Tollan A, Oian P, Kjeldsen SE, Eide I, Maltau JM (1993). "Progesterone reduces sympathetic tone without changing blood pressure or fluid balance in men". Gynecol. Obstet. Invest. 36 (4): 234–8. doi:10.1159/000292636. PMID 8300009.
- ^ Progesterone - Drugs.com, retrieved 2015-08-23
- ^ Josimovich J (11 November 2013). Gynecologic Endocrinology. Springer Science & Business Media. pp. 9, 25–29, 139. ISBN 978-1-4613-2157-6.
- ^ Jerome Frank Strauss; Robert L. Barbieri (2009). Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Elsevier Health Sciences. pp. 807–. ISBN 978-1-4160-4907-4.
- ^ Brady BM, Anderson RA, Kinniburgh D, Baird DT (2003). "Demonstration of progesterone receptor-mediated gonadotrophin suppression in the human male". Clin. Endocrinol. (Oxf). 58 (4): 506–12. doi:10.1046/j.1365-2265.2003.01751.x. PMID 12641635. S2CID 12567639.
- ^ a b c Heller CG, Moore DJ, Paulsen CA, Nelson WO, Laidlaw WM (December 1959). "Effects of progesterone and synthetic progestins on the reproductive physiology of normal men". Fed. Proc. 18: 1057–65. PMID 14400846. Archived from the original on 2018-12-18. Retrieved 2018-12-18.
- ^ Rothchild I (June 1957). "Effect of large doses of intravenously administered progesterone on gonadotropin excretion in the human female". J. Clin. Endocrinol. Metab. 17 (6): 754–9. doi:10.1210/jcem-17-6-754. PMID 13428841.
- ^ Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (25 August 2011). Campbell-Walsh Urology: Expert Consult Premium Edition: Enhanced Online Features and Print, 4-Volume Set. Elsevier Health Sciences. pp. 2938–. ISBN 978-1-4160-6911-9.
- ^ Kjeld JM, Puah CM, Kaufman B, Loizou S, Vlotides J, Gwee HM, Kahn F, Sood R, Joplin GF (1979). "Effects of norgestrel and ethinyloestradiol ingestion on serum levels of sex hormones and gonadotrophins in men". Clinical Endocrinology. 11 (5): 497–504. doi:10.1111/j.1365-2265.1979.tb03102.x. PMID 519881. S2CID 5836155.
- ^ a b Neumann, F.; Diallo, F.A.; Hasan, S.H.; Schenck, B.; Traore, I. (1976). "The Influence of Pharmaceutical Compounds on Male Fertility*". Andrologia. 8 (3): 203–235. doi:10.1111/j.1439-0272.1976.tb02137.x. ISSN 0303-4569. PMID 793446. S2CID 24859886.
- ^ Heller CG, Laidlaw WM, Harvey HT, Nelson WO (July 1958). "Effects of progestational compounds on the reproductive processes of the human male". Ann. N. Y. Acad. Sci. 71 (5): 649–65. doi:10.1111/j.1749-6632.1958.tb54641.x. PMID 13583821. S2CID 32637425.
- ^ a b Neumann, F. (1985). "Steroidal contraception — experimental background". Future Aspects in Contraception. pp. 129–144. doi:10.1007/978-94-009-4910-2_2. ISBN 978-94-010-8675-2.
- ^ Bain, J. (1980). "Androgen-Progestin Combinations: Clinical Trials". Regulation of Male Fertility. pp. 85–91. doi:10.1007/978-94-009-8875-0_9. ISBN 978-94-009-8877-4.
- ^ a b Petry, R.; Pfizenmayer, K. (1973). "Möglichkeiten der medikamentösen Fertilitätshemmung beim Mann". Deutsche Medizinische Wochenschrift. 98 (38): 1775–1779. doi:10.1055/s-0028-1107127. ISSN 0012-0472. PMID 4742513.
- ^ Sundsfjord JA, Aakvaag A, Norman N (August 1971). "Reduced plasma testosterone and LH in young men during progesterone administration". J. Reprod. Fertil. 26 (2): 263–5. doi:10.1530/jrf.0.0260263. PMID 5558416.
- ^ a b Turkmen S, Backstrom T, Wahlstrom G, Andreen L, Johansson IM (2011). "Tolerance to allopregnanolone with focus on the GABA-A receptor". Br. J. Pharmacol. 162 (2): 311–27. doi:10.1111/j.1476-5381.2010.01059.x. PMC 3031054. PMID 20883478.
- ^ a b Follesa P, Concas A, Porcu P, Sanna E, Serra M, Mostallino MC, Purdy RH, Biggio G (2001). "Role of allopregnanolone in regulation of GABA(A) receptor plasticity during long-term exposure to and withdrawal from progesterone". Brain Res. Brain Res. Rev. 37 (1–3): 81–90. doi:10.1016/s0165-0173(01)00125-4. PMID 11744076. S2CID 362309.
- ^ a b c Schiller CE, Schmidt PJ, Rubinow DR (2014). "Allopregnanolone as a mediator of affective switching in reproductive mood disorders". Psychopharmacology. 231 (17): 3557–67. doi:10.1007/s00213-014-3599-x. PMC 4135022. PMID 24846476.
- ^ a b c Bäckström T, Haage D, Löfgren M, Johansson IM, Strömberg J, Nyberg S, Andréen L, Ossewaarde L, van Wingen GA, Turkmen S, Bengtsson SK (2011). "Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons". Neuroscience. 191: 46–54. doi:10.1016/j.neuroscience.2011.03.061. PMID 21600269. S2CID 38928854.
- ^ Andréen L, Sundström-Poromaa I, Bixo M, Andersson A, Nyberg S, Bäckström T (February 2005). "Relationship between allopregnanolone and negative mood in postmenopausal women taking sequential hormone replacement therapy with vaginal progesterone". Psychoneuroendocrinology. 30 (2): 212–24. doi:10.1016/j.psyneuen.2004.07.003. PMID 15471618. S2CID 29760633.
Further reading
[edit]- Sitruk-Ware R, Bricaire C, De Lignieres B, Yaneva H, Mauvais-Jarvis P (October 1987). "Oral micronized progesterone. Bioavailability pharmacokinetics, pharmacological and therapeutic implications--a review". Contraception. 36 (4): 373–402. doi:10.1016/0010-7824(87)90088-6. PMID 3327648.
- Simon JA (December 1995). "Micronized progesterone: vaginal and oral uses". Clinical Obstetrics and Gynecology. 38 (4): 902–14. doi:10.1097/00003081-199538040-00024. PMID 8616985.
- Ruan X, Mueck AO (November 2014). "Systemic progesterone therapy--oral, vaginal, injections and even transdermal?". Maturitas. 79 (3): 248–55. doi:10.1016/j.maturitas.2014.07.009. PMID 25113944.